Why is it important that water is a universal solvent
Why Is It Important That Water Is A Universal Solvent. Because of this water is used as a solvent in many industries that make substances such as foods medicines fertilisers paints pesticides adhesives and paper. This is important to every living thing on earth. A solvent is simply a liquid that other substances can dissolve in and the reason that water has gained the label of universal solvent is because no other solvent can dissolve as many substances as it can. Water is called the universal solvent because it is capable of dissolving more substances than any other liquid.
 Ppt W A T E R Powerpoint Presentation Free Download Id 5173900 From slideserve.com
Ppt W A T E R Powerpoint Presentation Free Download Id 5173900 From slideserve.com
And water is called the universal solvent because it dissolves more substances than any other liquid. It is not a base or an acid and when distilled it does not conduct electricity one of few pure substances. Is water really a universal solvent. This helps water dissociate ionic compounds into their positive and negative ions. Water is a molecule which. The reason that this is true is because of some of water s unique properties.
Not only is water an important solvent but it is also an essential medium for living organisms.
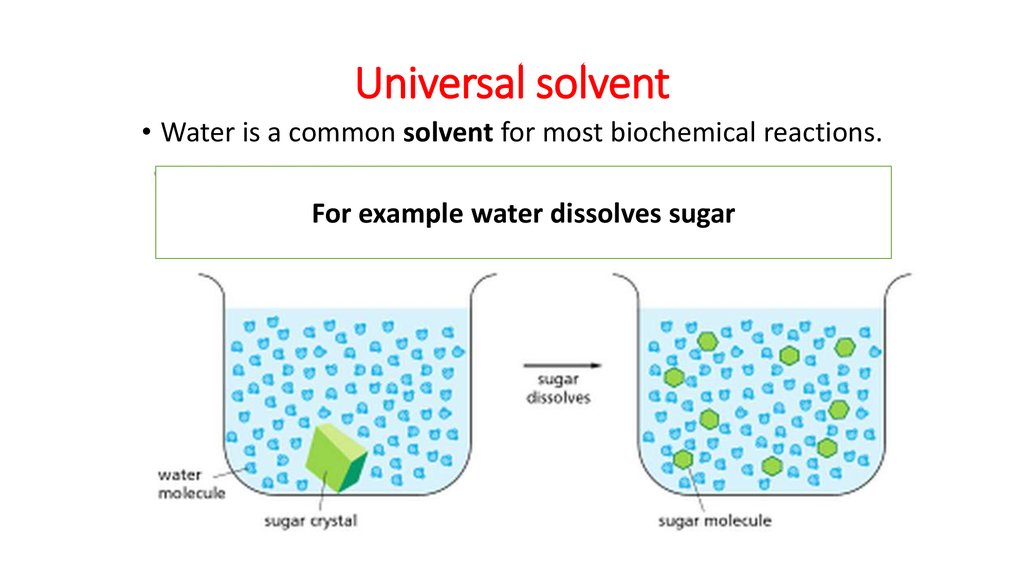
Water is the substance that we refer to as the universal solvent. And water is called the universal solvent because it dissolves more substances than any other liquid. Water is called the universal solvent because many materials are able to dissolve in water. As water stays as a liquid at the. It is important to mention that not all substances dissolve well in water for example oils. In terms of survival water does 2 important things.
 Source: slideplayer.com
Source: slideplayer.com
As water stays as a liquid at the. Is water really a universal solvent. It means that wherever water goes either through the ground or through our bodies it takes along valuable chemicals minerals and nutrients. Water is a molecule which. Water is called the universal solvent because it is capable of dissolving more substances than any other liquid.
 Source: thoughtco.com
Source: thoughtco.com
As water stays as a liquid at the. This has to do with the polarity of each water molecule. Water is called the universal solvent because many materials are able to dissolve in water. In terms of survival water does 2 important things. This is important to every living thing on earth.
 Source: ib.bioninja.com.au
Source: ib.bioninja.com.au
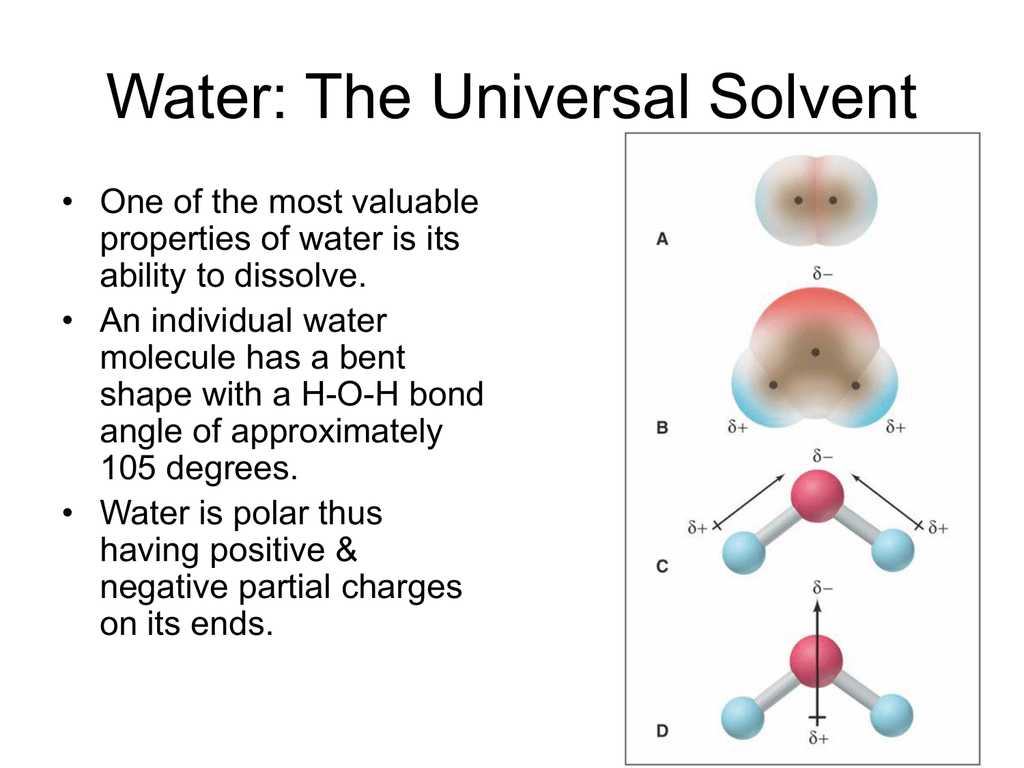
The hydrogen side of each water h 2 o molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge. The hydrogen side of each water h 2 o molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge. In terms of survival water does 2 important things. And water is called the universal solvent because it dissolves more substances than any other liquid. That s mostly what makes it so critical to life on earth.
 Source: studylib.net
Source: studylib.net
It is important to mention that not all substances dissolve well in water for example oils. It acts as a supporter and a heat holder. Water is the substance that we refer to as the universal solvent. This has to do with the polarity of each water molecule. In terms of survival water does 2 important things.
 Source: thoughtco.com
Source: thoughtco.com
It is important in two ways it is essential for survival it provides an environment for many species. This has to do with the polarity of each water molecule. Water is called the universal solvent because it is capable of dissolving more substances than any other liquid. Because of this water is used as a solvent in many industries that make substances such as foods medicines fertilisers paints pesticides adhesives and paper. The hydrogen side of each water h 2 o molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge.
 Source: usgs.gov
Source: usgs.gov
This is important to every living thing on earth. This is important to every living thing on earth. Is water really a universal solvent. It is important in two ways it is essential for survival it provides an environment for many species. The reason that this is true is because of some of water s unique properties.
 Source: m.youtube.com
Source: m.youtube.com
Is water really a universal solvent. Water is the substance that we refer to as the universal solvent. Because of this water is used as a solvent in many industries that make substances such as foods medicines fertilisers paints pesticides adhesives and paper. Water is called the universal solvent because more substances dissolve in water than in any other chemical. This helps water dissociate ionic compounds into their positive and negative ions.

It is not a base or an acid and when distilled it does not conduct electricity one of few pure substances. The hydrogen side of each water h 2 o molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge. Not only is water an important solvent but it is also an essential medium for living organisms. It is important in two ways it is essential for survival it provides an environment for many species. It is important to mention that not all substances dissolve well in water for example oils.
 Source: sciencenotes.org
Source: sciencenotes.org
It acts as a supporter and a heat holder. This is important to every living thing on earth. It is important in two ways it is essential for survival it provides an environment for many species. The reason that this is true is because of some of water s unique properties. The molecules of the oils have neither positive nor negative charge areas which is why they are not attracted to water molecules.
 Source: slideserve.com
Source: slideserve.com
This helps water dissociate ionic compounds into their positive and negative ions. This is important to every living thing on earth. The hydrogen side of each water h 2 o molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge. In terms of survival water does 2 important things. Is water really a universal solvent.
 Source: expii.com
Source: expii.com
Water is a universal solvent meaning it is able to dissolve a wide range of substances. It is important in two ways it is essential for survival it provides an environment for many species. This has to do with the polarity of each water molecule. Water is a universal solvent meaning it is able to dissolve a wide range of substances. Water is called the universal solvent because many materials are able to dissolve in water.
 Source: usgs.gov
Source: usgs.gov
The reason that this is true is because of some of water s unique properties. In terms of survival water does 2 important things. It means that wherever water goes either through the ground or through our bodies it takes along valuable chemicals minerals and nutrients. The molecules of the oils have neither positive nor negative charge areas which is why they are not attracted to water molecules. The reason that this is true is because of some of water s unique properties.
 Source: e-education.psu.edu
Source: e-education.psu.edu
The reason that this is true is because of some of water s unique properties. That s mostly what makes it so critical to life on earth. This is important to every living thing on earth. Water is called the universal solvent because many materials are able to dissolve in water. It is not a base or an acid and when distilled it does not conduct electricity one of few pure substances.
 Source: brainly.in
Source: brainly.in
It acts as a supporter and a heat holder. As water stays as a liquid at the. That s mostly what makes it so critical to life on earth. It is important in two ways it is essential for survival it provides an environment for many species. And water is called the universal solvent because it dissolves more substances than any other liquid.
 Source: en.ppt-online.org
Source: en.ppt-online.org
That s mostly what makes it so critical to life on earth. Water is a universal solvent meaning it is able to dissolve a wide range of substances. Water is called the universal solvent because more substances dissolve in water than in any other chemical. That s mostly what makes it so critical to life on earth. This has to do with the polarity of each water molecule.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why is it important that water is a universal solvent by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





