Why is an alloy stronger than a pure metal
Why Is An Alloy Stronger Than A Pure Metal. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. Gold is valuable and hence one of the reasons used in jewelry. Consequently pure metals are malleable and ductile. In a pure metal the layers of atoms can slide over eachother.
 Give At Least Two Reasons Alloys Are Better Than Pure Metals Brainly In From brainly.in
Give At Least Two Reasons Alloys Are Better Than Pure Metals Brainly In From brainly.in
In an alloy there are atoms of different sizes. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. The smaller or bigger atoms distort the layers of atoms in the pure metal. Alloys are used because they are often harder than pure metal. Consequently pure metals are malleable and ductile.
One reason that manufacturers combine pure metals to form alloys is to change the physical properties of the metals.
This means that a greater force is required for the layers to slide over. Gold is an obvious example. This is due to their atom arrangement. In an alloy there are atoms of different sizes. But when another element is added it shifts the atoms making the layers harder to shift. One reason that manufacturers combine pure metals to form alloys is to change the physical properties of the metals.
 Source: slideplayer.com
Source: slideplayer.com
One reason that manufacturers combine pure metals to form alloys is to change the physical properties of the metals. The smaller or bigger atoms distort the layers of atoms in the pure metal. Conversely alloys have lower electrical and thermal conductivity than pure metals. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. Gold is valuable and hence one of the reasons used in jewelry.
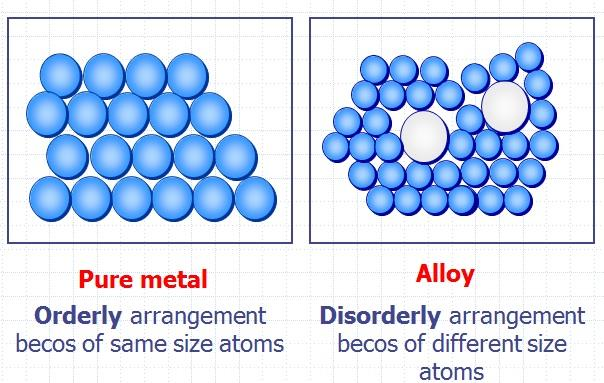 Source: app.emaze.com
Source: app.emaze.com
This makes it harder for the atoms to move around in metal alloys which is why they are typically much stronger and harder than pure metals. Making alloys usually involves mixing one harder element with a metal to create a stronger item. One reason that manufacturers combine pure metals to form alloys is to change the physical properties of the metals. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. Gold is an obvious example.
Source:
Or carbon are different sizes and they jam up the structure and stop the layers from sliding. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. The smaller or bigger atoms distort the layers of atoms in the pure metal. Alloy is a combo of 2 or extra metals. In an alloy there are atoms of different sizes.
 Source: chem2u.blogspot.com
Source: chem2u.blogspot.com
A pure metallic have an orderly association of atoms thus when a force is utilized the layers of atoms can be competent to slide over each different easily. This means that a greater force is required for the layers to slide over. Gold is an obvious example. Alloys are more commonly used than pure metals due to their increased hardness. An alloy is stronger than pure metal because in a pure metal all the atoms are the same size and ordered.
 Source: scienceshine.wordpress.com
Source: scienceshine.wordpress.com
Metal alloys are made up of differing atoms unlike pure metal where the atoms are all the same. An alloy is stronger than pure metal because in a pure metal all the atoms are the same size and ordered. Consequently pure metals are malleable and ductile. Alloys are used because they are often harder than pure metal. Pure metals may be too soft to hold up to regular use but alloying them makes them tougher.
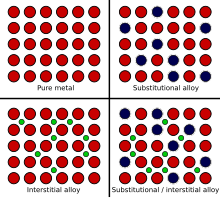 Source: en.wikipedia.org
Source: en.wikipedia.org
In a pure metal the layers of atoms can slide over eachother. But when another element is added it shifts the atoms making the layers harder to shift. In alloys the atoms are of varying sizes. The smaller or bigger atoms distort the layers of atoms in the pure metal. While most alloys are synthetic in rare instances they can also occur in nature.
 Source: pinterest.com
Source: pinterest.com
Pure metals may be too soft to hold up to regular use but alloying them makes them tougher. Alloy is a combo of 2 or extra metals. This means that a greater force is required for the layers to slide over. This makes it harder for the atoms to move around in metal alloys which is why they are typically much stronger and harder than pure metals. An alloy is stronger than pure metal because in a pure metal all the atoms are the same size and ordered.
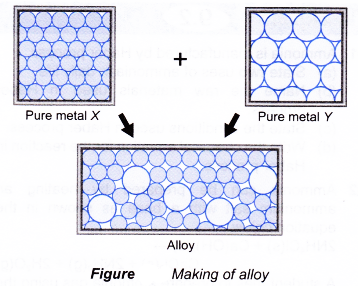 Source: aplustopper.com
Source: aplustopper.com
Conversely alloys have lower electrical and thermal conductivity than pure metals. Alloys are used because they are often harder than pure metal. Alloys are more commonly used than pure metals due to their increased hardness. Alloy is a combo of 2 or extra metals. Metal alloys are made up of differing atoms unlike pure metal where the atoms are all the same.
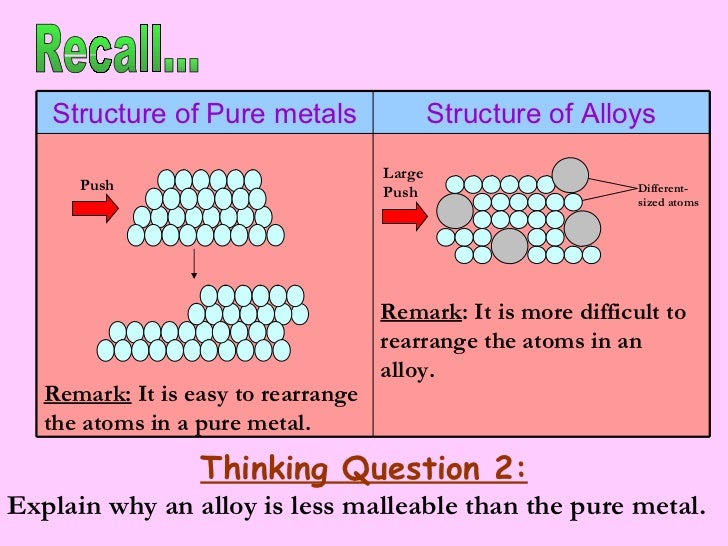 Source: slideshare.net
Source: slideshare.net
While most alloys are synthetic in rare instances they can also occur in nature. Gold is an obvious example. An alloy is stronger because the atoms if the added metal. This means that a greater force is required for the layers to slide over. Gold is valuable and hence one of the reasons used in jewelry.
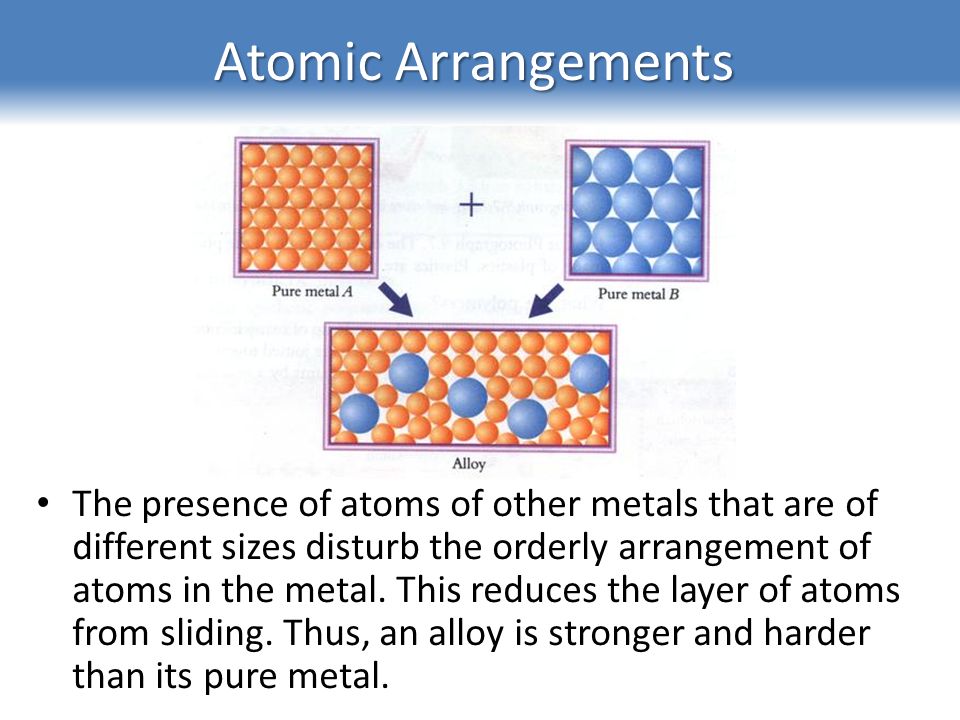 Source: slideplayer.com
Source: slideplayer.com
This makes it harder for the atoms to move around in metal alloys which is why they are typically much stronger and harder than pure metals. Consequently pure metals are malleable and ductile. Metal alloys are made up of differing atoms unlike pure metal where the atoms are all the same. In a pure metal the layers of atoms can slide over eachother. Alloys are more commonly used than pure metals due to their increased hardness.
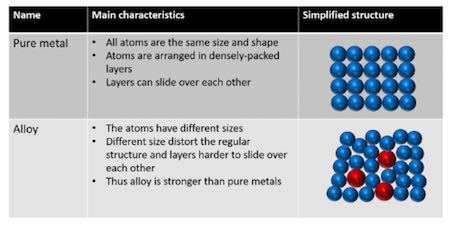 Source: library.tel-courses.org
Source: library.tel-courses.org
Why are alloys stronger than pure metals quizlet. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. This makes it harder for the atoms to move around in metal alloys which is why they are typically much stronger and harder than pure metals. Metal alloys are made up of differing atoms unlike pure metal where the atoms are all the same. Pure metals are typically soft so metals are taken and alloyed together to create a more durable compound.
 Source: brainly.in
Source: brainly.in
Making alloys usually involves mixing one harder element with a metal to create a stronger item. The smaller or bigger atoms distort the layers of atoms in the pure metal. While most alloys are synthetic in rare instances they can also occur in nature. This is due to their atom arrangement. One reason that manufacturers combine pure metals to form alloys is to change the physical properties of the metals.
 Source: tes.com
Source: tes.com
An alloy is stronger because the atoms if the added metal. One reason that manufacturers combine pure metals to form alloys is to change the physical properties of the metals. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. An alloy is stronger than pure metal because in a pure metal all the atoms are the same size and ordered. This means that a greater force is required for the layers to slide over.
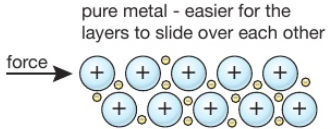 Source: tutormyself.com
Source: tutormyself.com
A pure metallic have an orderly association of atoms thus when a force is utilized the layers of atoms can be competent to slide over each different easily. Conversely alloys have lower electrical and thermal conductivity than pure metals. Consequently pure metals are malleable and ductile. Alloy is a combo of 2 or extra metals. Alloys are used because they are often harder than pure metal.
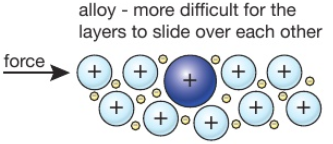 Source: tutormyself.com
Source: tutormyself.com
But when another element is added it shifts the atoms making the layers harder to shift. This makes it harder for the atoms to move around in metal alloys which is why they are typically much stronger and harder than pure metals. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. Alloy is a combo of 2 or extra metals. Conversely alloys have lower electrical and thermal conductivity than pure metals.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why is an alloy stronger than a pure metal by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





