Why does salt water conduct electricity
Why Does Salt Water Conduct Electricity. Saltwater is a good conductor of electricity because it is an electrolyte solution. An ion is an atom or molecule with a net electric charge due to the loss or gain of one or more electrons. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. Salt water contains impurities when salt or sodium chloride dissolved in water it separates into ions sodium ion and chloride ion.
 Salt Pools The Truth About Salt Generated Chlorine From blog.orendatech.com
Salt Pools The Truth About Salt Generated Chlorine From blog.orendatech.com
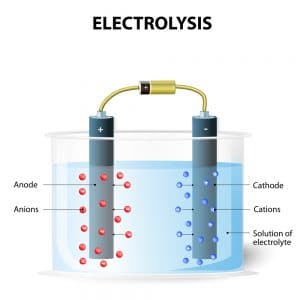
Salt is an ionic compound which is made up of sodium ions and chlorine ions. When salts are dissolved in water they separate into different electrically charged atoms called ions. An ion is an atom or molecule with a net electric charge due to the loss or gain of one or more electrons. Electricity is a steady flow of electrons or electrically charged particles through a substance. When sodium chloride dissolves in water the water separates the sodium and chlorine ions. Saltwater is a good conductor of electricity because it is an electrolyte solution.
These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl.
Substances such as salts acids and hydroxides that also are electrolytes can conduct electric current. Salt molecules are made of sodium ions and chlorine ions. Saltwater is a mixture that consists of water and sodium chloride. Salt water contains impurities when salt or sodium chloride dissolved in water it separates into ions sodium ion and chloride ion. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. Substances such as salts acids and hydroxides that also are electrolytes can conduct electric current.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
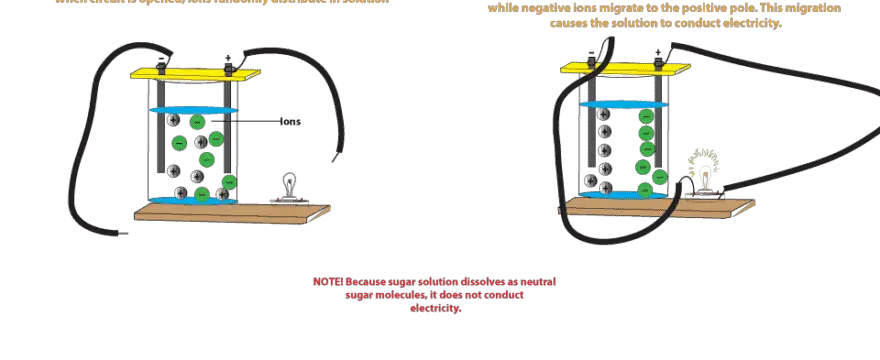
Salt molecules are made of sodium ions and chlorine ions. An ion is an atom or molecule with a net electric charge due to the loss or gain of one or more electrons. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. Electricity is a steady flow of electrons or electrically charged particles through a substance. When sodium chloride dissolves in water the water separates the sodium and chlorine ions.
 Source: slideplayer.com
Source: slideplayer.com
Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. When sodium chloride dissolves in water the water separates the sodium and chlorine ions. Any impurities like salts in the water enable it to conduct electricity. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. Why does salt water conduct electricity.
 Source: pinterest.com
Source: pinterest.com
This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy. Salt molecules are made of sodium ions and chlorine ions. Saltwater is a mixture that consists of water and sodium chloride. Salt water contains impurities when salt or sodium chloride dissolved in water it separates into ions sodium ion and chloride ion. Salt is an ionic compound which is made up of sodium ions and chlorine ions.
 Source: kidder.ca
Source: kidder.ca
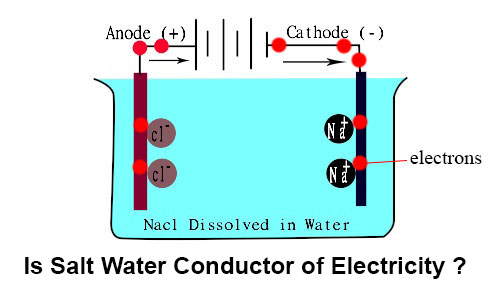
Salt water contains impurities when salt or sodium chloride dissolved in water it separates into ions sodium ion and chloride ion. These free mobile ions can conduct electricity thus making salt water conduct electricity. When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. Electricity is a steady flow of electrons or electrically charged particles through a substance. To understand why salt water conducts electricity we have to first understand what electricity is.
 Source: slideplayer.com
Source: slideplayer.com
When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. When sodium chloride dissolves in water the water separates the sodium and chlorine ions. When salts are dissolved in water they separate into different electrically charged atoms called ions. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions.
 Source: diy.smartkids123.com
Source: diy.smartkids123.com
Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. An ion is an atom or molecule with a net electric charge due to the loss or gain of one or more electrons.
 Source: m.youtube.com
Source: m.youtube.com
When sodium chloride dissolves in water the water separates the sodium and chlorine ions. When salts are dissolved in water they separate into different electrically charged atoms called ions. Why does salt water conduct electricity. An ion is an atom that has an electrical charge because it has either gained or lost an electron also meaning it has a positive charge and a negative charge when you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. Saltwater is a mixture that consists of water and sodium chloride.
Source: msestudent.com
An ion is an atom that has an electrical charge because it has either gained or lost an electron also meaning it has a positive charge and a negative charge when you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely. When salts are dissolved in water they separate into different electrically charged atoms called ions. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. Saltwater is a good conductor of electricity because it is an electrolyte solution. These free mobile ions can conduct electricity thus making salt water conduct electricity.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Electricity is a steady flow of electrons or electrically charged particles through a substance. To understand why salt water conducts electricity we have to first understand what electricity is. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. When salts are dissolved in water they separate into different electrically charged atoms called ions.
 Source: youtube.com
Source: youtube.com
Salt water contains impurities when salt or sodium chloride dissolved in water it separates into ions sodium ion and chloride ion. Salt molecules are made of sodium ions and chlorine ions. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. An ion is an atom or molecule with a net electric charge due to the loss or gain of one or more electrons. An ion is an atom that has an electrical charge because it has either gained or lost an electron also meaning it has a positive charge and a negative charge when you put salt in water the water molecules pull the sodium and chlorine ions apart so they are floating freely.
 Source: blog.orendatech.com
Source: blog.orendatech.com
To understand why salt water conducts electricity we have to first understand what electricity is. When sodium chloride dissolves in water the water separates the sodium and chlorine ions. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions.
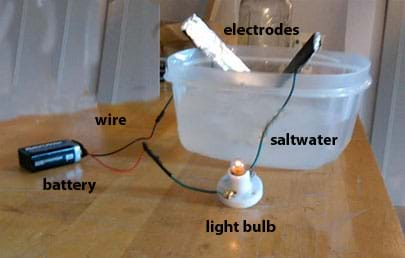 Source: teachengineering.org
Source: teachengineering.org
Saltwater is a good conductor of electricity because it is an electrolyte solution. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. Salt is an ionic compound which is made up of sodium ions and chlorine ions. Any impurities like salts in the water enable it to conduct electricity. Why does salt water conduct electricity.
 Source: physics.stackexchange.com
Source: physics.stackexchange.com
Any impurities like salts in the water enable it to conduct electricity. Salt is an ionic compound which is made up of sodium ions and chlorine ions. When table salt nacl is dissolved in water the water molecules pull the sodium na and chlorine cl ions apart. Why does salt water conduct electricity. When salts are dissolved in water they separate into different electrically charged atoms called ions.
Source: homesciencetools.com
This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy. Salt or sodium chloride nacl breaks up into positive na ions and negative cl ions. This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy. When sodium chloride dissolves in water the water separates the sodium and chlorine ions. Saltwater is a good conductor of electricity because it is an electrolyte solution.
 Source: otbanvali.over-blog.com
Source: otbanvali.over-blog.com
Salt is an ionic compound which is made up of sodium ions and chlorine ions. Substances such as salts acids and hydroxides that also are electrolytes can conduct electric current. Why does salt water conduct electricity. When salts are dissolved in water they separate into different electrically charged atoms called ions. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why does salt water conduct electricity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.