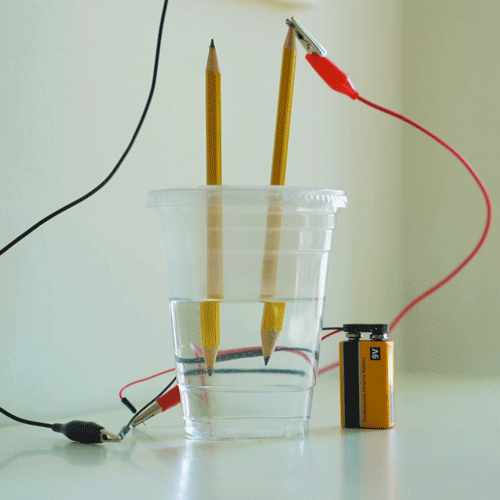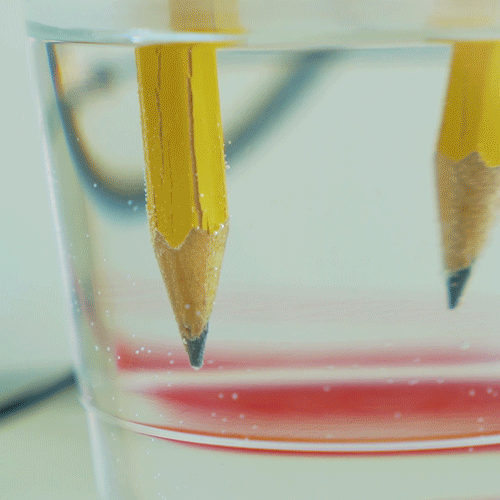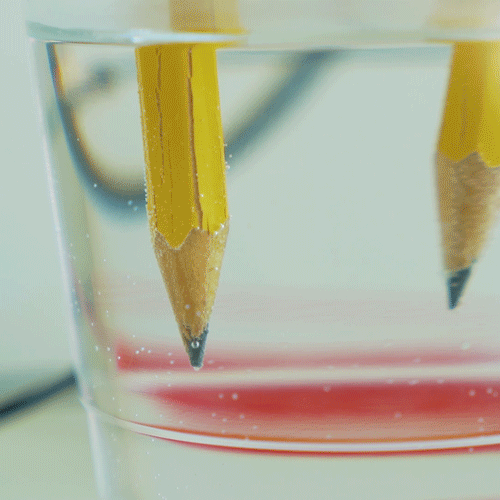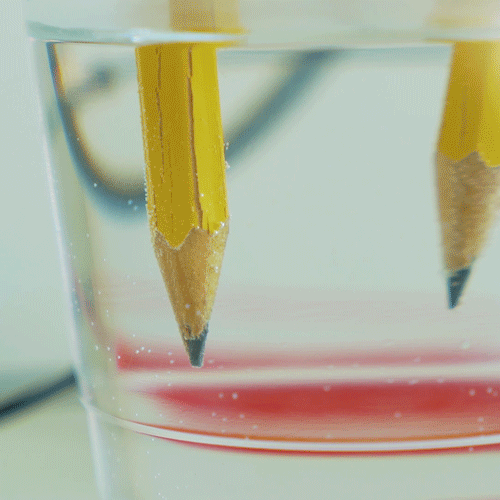
Why do we electroplate objects
Why Do We Electroplate Objects. Once this occurs the metal particles bind to the surface of the object. And the anode is usually either a block of that metal or of some inert conductive material. Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. The electrolyte is a solution of a salt of the metal to be coated.
 What Is Electroplating Justscience From justscience.in
What Is Electroplating Justscience From justscience.in
And the anode is usually either a block of that metal or of some inert conductive material. The industrial use of electroplating is also a popular choice in businesses when corrosion against protection is necessary to prevent the premature demise of metal materials. The electrolyte is a solution of a salt of the metal to be coated. Chromium plating improves the appearance of objects and also improves its wear. Electroplating allows us to make parts of strong flexible inexpensive materials like steel while having surfaces of hard brittle materials like chromium or nickel diamond composites so that we can have molds and cutting tools and machine parts and truck bumpers that will wear well while not being subject to fracturing. Electroplating is widely used in indus.
Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals.
Electroplating is widely used in indus. It s then treated in various chemicals including a catalytic solution to induce oxidation. Whether things are plated for decoration or protection the thickness of the plated layer is another important consideration. Purpose of electroplating there are several reasons why you might want to coat a conductive surface with metal. Electroplating allows us to make parts of strong flexible inexpensive materials like steel while having surfaces of hard brittle materials like chromium or nickel diamond composites so that we can have molds and cutting tools and machine parts and truck bumpers that will wear well while not being subject to fracturing. Electroless plating is a simpler plating process that eliminates the need for an electric current.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Electroplating electrolysis is used to electroplate objects coat them with a thin layer or metal. Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. The industrial use of electroplating is also a popular choice in businesses when corrosion against protection is necessary to prevent the premature demise of metal materials. And the anode is usually either a block of that metal or of some inert conductive material. It s then treated in various chemicals including a catalytic solution to induce oxidation.
 Source: ecfinc.com
Source: ecfinc.com
Purpose of electroplating there are several reasons why you might want to coat a conductive surface with metal. The current is provided by an external power supply. Electroplating is spectacular at enhancing the appearance of the surface of a metal and because of this jewellery is often electroplated with an extremely thin layer of precious metal to make it more appealing and attractive to potential customers. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. Electroless plating is a simpler plating process that eliminates the need for an electric current.
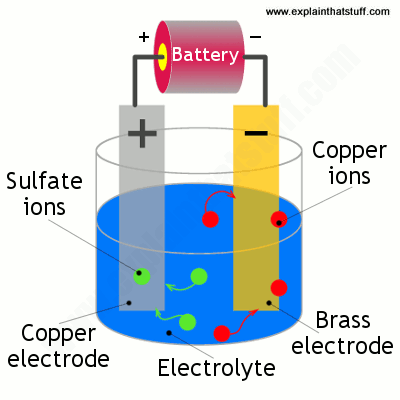 Source: explainthatstuff.com
Source: explainthatstuff.com
Electroplating is widely used in indus. The industrial use of electroplating is also a popular choice in businesses when corrosion against protection is necessary to prevent the premature demise of metal materials. Electroplating is widely used in indus. Electroplating is spectacular at enhancing the appearance of the surface of a metal and because of this jewellery is often electroplated with an extremely thin layer of precious metal to make it more appealing and attractive to potential customers. This is useful for coating a cheaper metal with a more expensive one such as copper or silver.
 Source: explainthatstuff.com
Source: explainthatstuff.com
Electroplating is spectacular at enhancing the appearance of the surface of a metal and because of this jewellery is often electroplated with an extremely thin layer of precious metal to make it more appealing and attractive to potential customers. It s then treated in various chemicals including a catalytic solution to induce oxidation. And the anode is usually either a block of that metal or of some inert conductive material. Electroplating is spectacular at enhancing the appearance of the surface of a metal and because of this jewellery is often electroplated with an extremely thin layer of precious metal to make it more appealing and attractive to potential customers. The electrolyte is a solution of a salt of the metal to be coated.
 Source: slideplayer.com
Source: slideplayer.com
Purpose of electroplating there are several reasons why you might want to coat a conductive surface with metal. As with electroplating the object is first cleaned. This is useful for coating a cheaper metal with a more expensive one such as copper or silver. The electrolyte is a solution of a salt of the metal to be coated. Nickel plating tin plating and their various alloys are all used for corrosion protection on nuts bolts housings brackets and many other metal parts and components.
 Source: santoshelectroplaters.in
Source: santoshelectroplaters.in
And the anode is usually either a block of that metal or of some inert conductive material. And the anode is usually either a block of that metal or of some inert conductive material. Once this occurs the metal particles bind to the surface of the object. Electroplating electrolysis is used to electroplate objects coat them with a thin layer or metal. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current.
 Source: studiousguy.com
Source: studiousguy.com
Purpose of electroplating there are several reasons why you might want to coat a conductive surface with metal. The industrial use of electroplating is also a popular choice in businesses when corrosion against protection is necessary to prevent the premature demise of metal materials. Once this occurs the metal particles bind to the surface of the object. Nickel plating tin plating and their various alloys are all used for corrosion protection on nuts bolts housings brackets and many other metal parts and components. Electroplating allows us to make parts of strong flexible inexpensive materials like steel while having surfaces of hard brittle materials like chromium or nickel diamond composites so that we can have molds and cutting tools and machine parts and truck bumpers that will wear well while not being subject to fracturing.
 Source: studiousguy.com
Source: studiousguy.com
Electroplating electrolysis is used to electroplate objects coat them with a thin layer or metal. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. How thick is electroplating. Silver plating and gold plating of jewelry or silverware typically are performed to improve the appearance and value of the items. Once this occurs the metal particles bind to the surface of the object.
 Source: bendplating.com
Source: bendplating.com
Electroplating is spectacular at enhancing the appearance of the surface of a metal and because of this jewellery is often electroplated with an extremely thin layer of precious metal to make it more appealing and attractive to potential customers. Electroplating is widely used in indus. Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. Nickel plating tin plating and their various alloys are all used for corrosion protection on nuts bolts housings brackets and many other metal parts and components. It s then treated in various chemicals including a catalytic solution to induce oxidation.
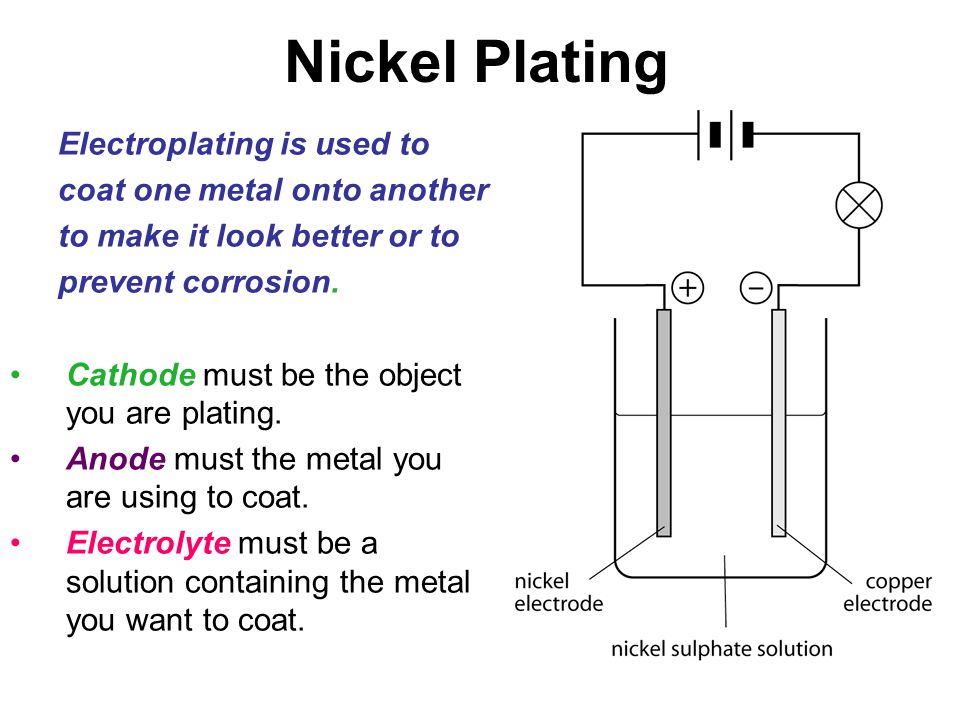 Source: slideplayer.com
Source: slideplayer.com
The industrial use of electroplating is also a popular choice in businesses when corrosion against protection is necessary to prevent the premature demise of metal materials. The electrolyte is a solution of a salt of the metal to be coated. Electroless plating is a simpler plating process that eliminates the need for an electric current. It s then treated in various chemicals including a catalytic solution to induce oxidation. Whether things are plated for decoration or protection the thickness of the plated layer is another important consideration.
 Source: thoughtco.com
Source: thoughtco.com
Whether things are plated for decoration or protection the thickness of the plated layer is another important consideration. Electroplating allows us to make parts of strong flexible inexpensive materials like steel while having surfaces of hard brittle materials like chromium or nickel diamond composites so that we can have molds and cutting tools and machine parts and truck bumpers that will wear well while not being subject to fracturing. And the anode is usually either a block of that metal or of some inert conductive material. As with electroplating the object is first cleaned. Whether things are plated for decoration or protection the thickness of the plated layer is another important consideration.
 Source: thoughtco.com
Source: thoughtco.com
Nickel plating tin plating and their various alloys are all used for corrosion protection on nuts bolts housings brackets and many other metal parts and components. How thick is electroplating. And the anode is usually either a block of that metal or of some inert conductive material. The current is provided by an external power supply. The electrolyte is a solution of a salt of the metal to be coated.
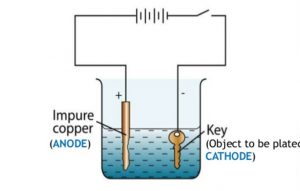 Source: classnotes.org.in
Source: classnotes.org.in
Electroplating is widely used in indus. Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. And the anode is usually either a block of that metal or of some inert conductive material. Once this occurs the metal particles bind to the surface of the object. Silver plating and gold plating of jewelry or silverware typically are performed to improve the appearance and value of the items.
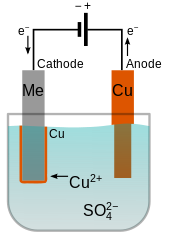 Source: en.wikipedia.org
Source: en.wikipedia.org
The current is provided by an external power supply. Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. Purpose of electroplating there are several reasons why you might want to coat a conductive surface with metal. Electroplating electrolysis is used to electroplate objects coat them with a thin layer or metal. The part to be coated acts as the cathode of an electrolytic cell.
 Source: justscience.in
Source: justscience.in
Electroplating allows us to make parts of strong flexible inexpensive materials like steel while having surfaces of hard brittle materials like chromium or nickel diamond composites so that we can have molds and cutting tools and machine parts and truck bumpers that will wear well while not being subject to fracturing. Electroplating is spectacular at enhancing the appearance of the surface of a metal and because of this jewellery is often electroplated with an extremely thin layer of precious metal to make it more appealing and attractive to potential customers. The electrolyte is a solution of a salt of the metal to be coated. Electroplating electrolysis is used to electroplate objects coat them with a thin layer or metal. Purpose of electroplating there are several reasons why you might want to coat a conductive surface with metal.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why do we electroplate objects by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.