Why are alloys harder than pure metals
Why Are Alloys Harder Than Pure Metals. This makes it more difficult for the layers to slide over each other so alloys are harder than the pure metal. They can be made harder by adding another element to the pure metal so forming an alloy. Why alloys are harder than pure metalsmaterials are harder the more difficult it is to make their atoms slide past each other. This explains why an alloy often has more uses than the pure elements it is made from.
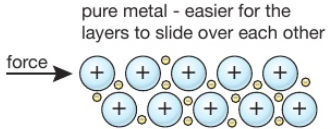 2 27 Triple Only Explain Why Alloys Are Harder Than Pure Metals Tutormyself Chemistry From tutormyself.com
2 27 Triple Only Explain Why Alloys Are Harder Than Pure Metals Tutormyself Chemistry From tutormyself.com
This breaks up the regular lattice arrangement and makes it more difficult for layers of ions to slide over each other. They can be made harder by adding another element to the pure metal so forming an alloy. This explains why an alloy often has more uses than the pure elements it is made from. While most alloys are synthetic in rare instances they can also occur in nature. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. Alloys contain atoms of different sizes.
Conversely alloys have lower electrical and thermal conductivity than pure metals.
In an alloy the different elements have slightly different sized atoms. Why alloys are harder than pure metalsmaterials are harder the more difficult it is to make their atoms slide past each other. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. While most alloys are synthetic in rare instances they can also occur in nature. People also ask why are alloys harder than pure metals gcse. This explains why an alloy often has more uses than the pure elements it is made from.
Source:
In an alloy the different elements have slightly different sized atoms. This is relatively easy in pure metals because all atoms are the same. While most alloys are synthetic in rare instances they can also occur in nature. This breaks up the regular lattice arrangement and makes it more difficult for layers of ions to slide over each other. Copper gold and aluminium are too soft for many uses.
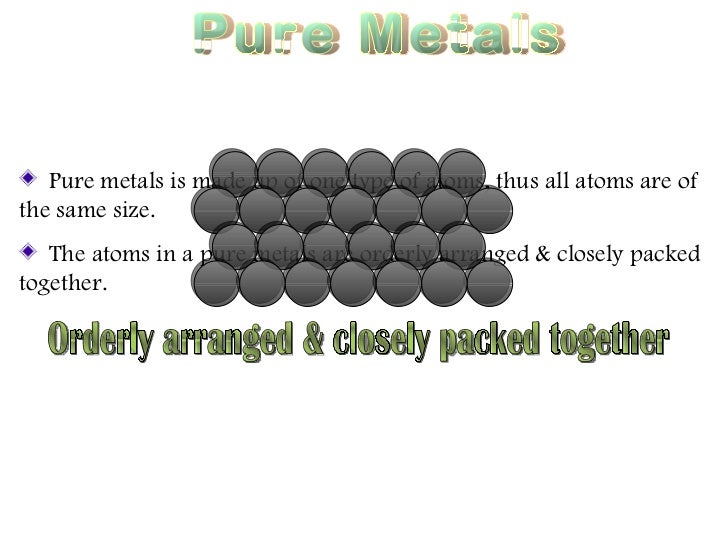 Source: slideshare.net
Source: slideshare.net
It is more difficult for layers of atoms to slide over each other in alloys. It is more difficult for layers of atoms to slide over each other in alloys. People also ask why are alloys harder than pure metals gcse. Copper gold and aluminium are too soft for many uses. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another.
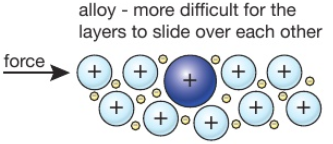 Source: tutormyself.com
Source: tutormyself.com
People also ask why are alloys harder than pure metals gcse. This explains why an alloy often has more uses than the pure elements it is made from. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. It is more difficult for layers of atoms to slide over each other in alloys. They can be made harder by adding another element to the pure metal so forming an alloy.
 Source: tutormyself.com
Source: tutormyself.com
People also ask why are alloys harder than pure metals gcse. This makes it more difficult for the layers to slide over each other so alloys are harder than the pure metal. Alloys are harder than the individual pure metals from which they are made. While most alloys are synthetic in rare instances they can also occur in nature. Alloys contain atoms of different sizes.
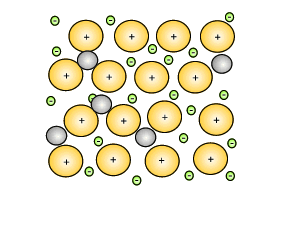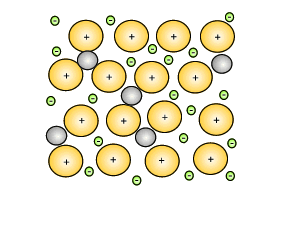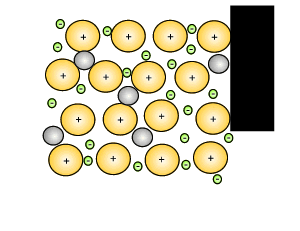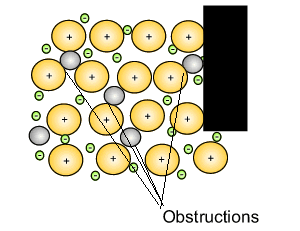 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Alloys are harder than the individual pure metals from which they are made. People also ask why are alloys harder than pure metals gcse. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. Alloys are harder than the individual pure metals from which they are made. While most alloys are synthetic in rare instances they can also occur in nature.
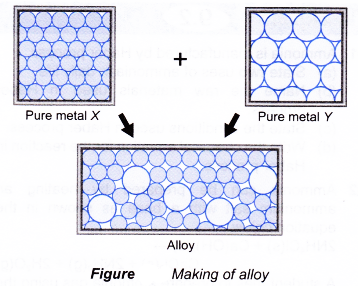 Source: aplustopper.com
Source: aplustopper.com
This explains why an alloy often has more uses than the pure elements it is made from. Copper gold and aluminium are too soft for many uses. Conversely alloys have lower electrical and thermal conductivity than pure metals. In an alloy the different elements have slightly different sized atoms. This makes it more difficult for the layers to slide over each other so alloys are harder than the pure metal.
 Source: slideplayer.com
Source: slideplayer.com
Copper gold and aluminium are too soft for many uses. In an alloy the different elements have slightly different sized atoms. This is relatively easy in pure metals because all atoms are the same. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. This explains why an alloy often has more uses than the pure elements it is made from.
 Source: brainly.in
Source: brainly.in
They can be made harder by adding another element to the pure metal so forming an alloy. Alloys contain atoms of different sizes. People also ask why are alloys harder than pure metals gcse. This is relatively easy in pure metals because all atoms are the same. This breaks up the regular lattice arrangement and makes it more difficult for layers of ions to slide over each other.
 Source: scienceshine.wordpress.com
Source: scienceshine.wordpress.com
This explains why an alloy often has more uses than the pure elements it is made from. Alloys contain atoms of different sizes. This makes it more difficult for the layers to slide over each other so alloys are harder than the pure metal. It is more difficult for layers of atoms to slide over each other in alloys. In an alloy the different elements have slightly different sized atoms.
 Source: pinterest.com
Source: pinterest.com
Copper gold and aluminium are too soft for many uses. This is relatively easy in pure metals because all atoms are the same. This breaks up the regular lattice arrangement and makes it more difficult for layers of ions to slide over each other. Why alloys are harder than pure metalsmaterials are harder the more difficult it is to make their atoms slide past each other. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another.
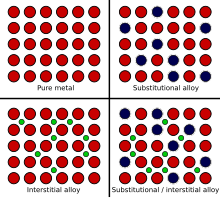 Source: en.wikipedia.org
Source: en.wikipedia.org
Alloys contain atoms of different sizes. While most alloys are synthetic in rare instances they can also occur in nature. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. They can be made harder by adding another element to the pure metal so forming an alloy. Copper gold and aluminium are too soft for many uses.
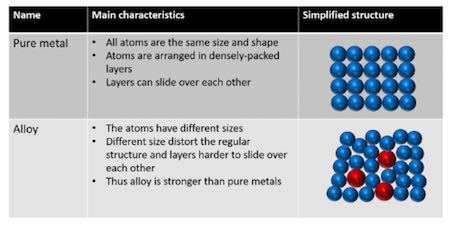 Source: library.tel-courses.org
Source: library.tel-courses.org
Copper gold and aluminium are too soft for many uses. Alloys are harder than pure metals because their molecular structure prevents the metal atoms from sliding over one another. This is relatively easy in pure metals because all atoms are the same. Conversely alloys have lower electrical and thermal conductivity than pure metals. In an alloy the different elements have slightly different sized atoms.
 Source: tes.com
Source: tes.com
Copper gold and aluminium are too soft for many uses. This breaks up the regular lattice arrangement and makes it more difficult for layers of ions to slide over each other. Copper gold and aluminium are too soft for many uses. This explains why an alloy often has more uses than the pure elements it is made from. While most alloys are synthetic in rare instances they can also occur in nature.
Source: igcse-chemistry-2017.blogspot.com
This makes it more difficult for the layers to slide over each other so alloys are harder than the pure metal. This makes it more difficult for the layers to slide over each other so alloys are harder than the pure metal. Alloys contain atoms of different sizes. This explains why an alloy often has more uses than the pure elements it is made from. People also ask why are alloys harder than pure metals gcse.
Source: sites.google.com
Conversely alloys have lower electrical and thermal conductivity than pure metals. Conversely alloys have lower electrical and thermal conductivity than pure metals. Alloys contain atoms of different sizes. While most alloys are synthetic in rare instances they can also occur in nature. Why alloys are harder than pure metalsmaterials are harder the more difficult it is to make their atoms slide past each other.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title why are alloys harder than pure metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






