Why are alloys better than metals
Why Are Alloys Better Than Metals. Alloys are more commonly used than pure metals due to their increased hardness. The benefits of inconel alloys. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. The advantage of alloys differs depending on the specific alloy.
 Give At Least Two Reasons Alloys Are Better Than Pure Metals Brainly In From brainly.in
Give At Least Two Reasons Alloys Are Better Than Pure Metals Brainly In From brainly.in
This is why car wheels are made of alloys so that they dont react with air or water. Alloys provide additional strength to a metal s structure because of the differing sizes of atoms in the alloy. Alloys are used because they are often harder than pure metal. The alloy is harder and stronger than. This means that the layers of atoms cannot slide over each other easily making the whole alloy much stronger than any of the pure metals that the alloy contains in isolation. Published in physical review letters this study explains why nanoparticles made with an alloy of metals help to synthetize longer cnts compared with conventional monometallic catalysts.
Almost all real world metals are alloys instead of pure metals.
Alloys are more commonly used than pure metals due to their increased hardness. This increases its hardness. In alloys the atoms are of varying sizes. Alloys provide additional strength to a metal s structure because of the differing sizes of atoms in the alloy. This means that the layers of atoms cannot slide over each other easily making the whole alloy much stronger than any of the pure metals that the alloy contains in isolation. Alloy wheels are made of an alloy of light metals namely aluminium nickel magnesium or a combination of these metals.
 Source: brainly.in
Source: brainly.in
Alloy wheels are made of an alloy of light metals namely aluminium nickel magnesium or a combination of these metals. The alloy is harder and stronger than. Summary metal vs alloy. The advantage of alloys differs depending on the specific alloy. Another difference between metal and alloy is that unlike pure metals alloy does not easily undergo chemical reactions with air and water which is why we tend to use alloys in car wheels rather than pure metal.
 Source: differencebetween.com
Source: differencebetween.com
This is due to their atom arrangement. Some are better than pure materials while some are worse. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. Alloys are used because they are often harder than pure metal. Summary metal vs alloy.
 Source: pinterest.com
Source: pinterest.com
The most important downside of alloys is that they have lower conductivity than pure metals which is why the only pure metal you encounter in daily life is copper in electrical wires. The alloy is harder and stronger than. The benefits of inconel alloys. Less weight also means less strain on suspension components. Published in physical review letters this study explains why nanoparticles made with an alloy of metals help to synthetize longer cnts compared with conventional monometallic catalysts.
 Source: slideplayer.com
Source: slideplayer.com
In alloys the atoms are of varying sizes. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. Alloy is a subcategory of metal. This is why car wheels are made of alloys so that they dont react with air or water. Less weight also means less strain on suspension components.
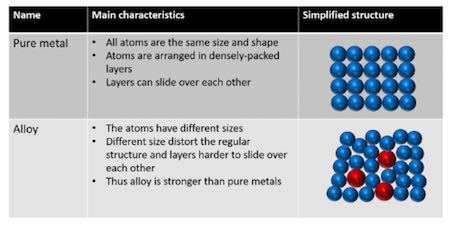 Source: library.tel-courses.org
Source: library.tel-courses.org
Some are better than pure materials while some are worse. In alloys the atoms are of varying sizes. Published in physical review letters this study explains why nanoparticles made with an alloy of metals help to synthetize longer cnts compared with conventional monometallic catalysts. The key difference between metal and alloy is that the metal is a pure. This is why car wheels are made of alloys so that they dont react with air or water.
 Source: slideplayer.com
Source: slideplayer.com
Alloys are used because they are often harder than pure metal. Alloys provide additional strength to a metal s structure because of the differing sizes of atoms in the alloy. Alloys are more commonly used than pure metals due to their increased hardness. Compared to pure metals alloys can be stronger more resistant to damage and more versatile. Some are better than pure materials while some are worse.
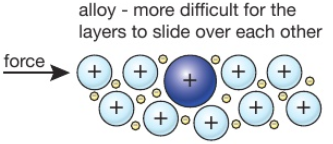 Source: tutormyself.com
Source: tutormyself.com
The key difference between metal and alloy is that the metal is a pure. The smaller or bigger atoms distort the layers of atoms in the pure metal. Summary metal vs alloy. This increases its hardness. Alloy wheels are made of an alloy of light metals namely aluminium nickel magnesium or a combination of these metals.
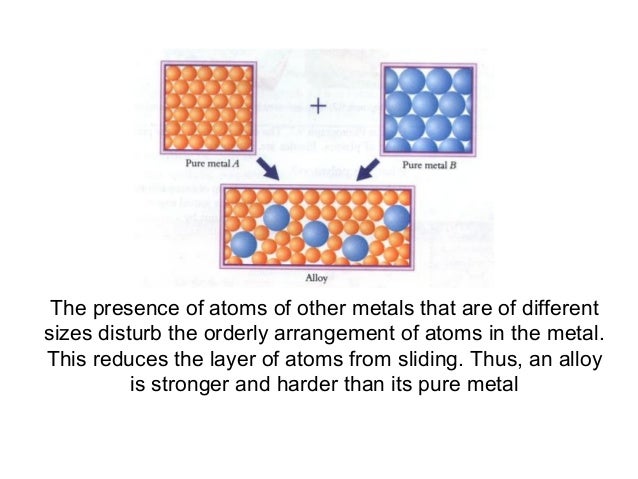 Source: slideshare.net
Source: slideshare.net
The advantage of alloys differs depending on the specific alloy. Alloys are more commonly used than pure metals due to their increased hardness. Most alloys are formed for one or two specific properties like strength and rust resistance. This increases its hardness. This is why car wheels are made of alloys so that they dont react with air or water.
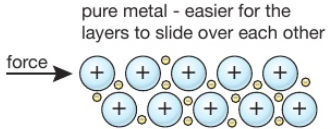 Source: tutormyself.com
Source: tutormyself.com
In alloys the atoms are of varying sizes. Alloys also tend to have better corrosion resistance than pure metals and are more versatile for manipulating into different forms. The alloy is harder and stronger than. Making alloys usually involves mixing one harder element with a metal to create a stronger item. Almost all real world metals are alloys instead of pure metals.
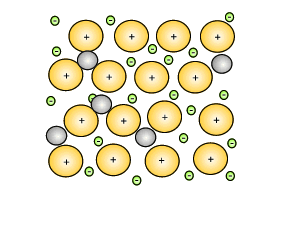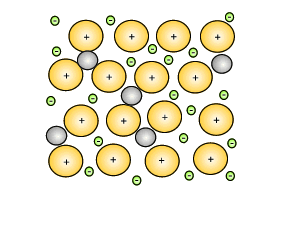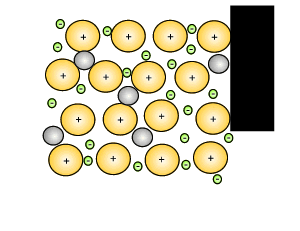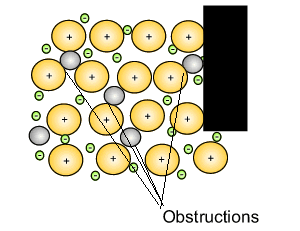 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Another difference between metal and alloy is that unlike pure metals alloy does not easily undergo chemical reactions with air and water which is why we tend to use alloys in car wheels rather than pure metal. Making alloys usually involves mixing one harder element with a metal to create a stronger item. Published in physical review letters this study explains why nanoparticles made with an alloy of metals help to synthetize longer cnts compared with conventional monometallic catalysts. Some are better than pure materials while some are worse. The most important downside of alloys is that they have lower conductivity than pure metals which is why the only pure metal you encounter in daily life is copper in electrical wires.

In alloys the atoms are of varying sizes. Making alloys usually involves mixing one harder element with a metal to create a stronger item. In alloys the atoms are of varying sizes. The alloy is harder and stronger than. Among other advantages alloys are usually stronger and have a lower melting point than pure metals.
 Source: tes.com
Source: tes.com
The benefits of inconel alloys. This increases its hardness. The smaller or bigger atoms distort the layers of atoms in the pure metal. Summary metal vs alloy. The alloy is harder and stronger than.

This increases its hardness. The alloy is harder and stronger than. Alloy is a subcategory of metal. In alloys the atoms are of varying sizes. The smaller or bigger atoms distort the layers of atoms in the pure metal.
 Source: mse.umd.edu
Source: mse.umd.edu
Therefore metal is a natural substance while alloy is a man made substance. The advantage of alloys differs depending on the specific alloy. Alloys provide additional strength to a metal s structure because of the differing sizes of atoms in the alloy. Alloy is a subcategory of metal. Making alloys usually involves mixing one harder element with a metal to create a stronger item.
 Source: slideplayer.com
Source: slideplayer.com
Metals are very important substances that we use in our day to day life. This increases its hardness. The most important downside of alloys is that they have lower conductivity than pure metals which is why the only pure metal you encounter in daily life is copper in electrical wires. Alloys are used because they are often harder than pure metal. This is due to their atom arrangement.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title why are alloys better than metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





