Which metal gets coated during electroplating
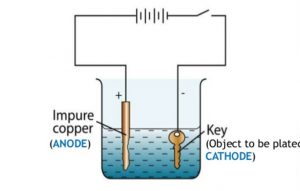
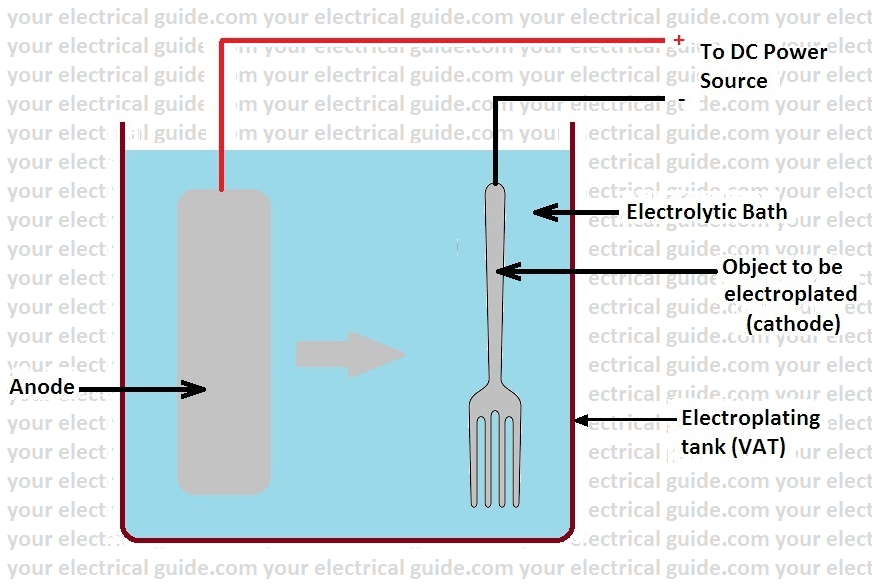
Which Metal Gets Coated During Electroplating. A kinds of metals can be electroplated such as gold silver tin zinc copper cadmium. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The metal that is the cathode gets coated during electroplating. The current is provided by an external power supply.
 Electroplating Britannica From britannica.com
Electroplating Britannica From britannica.com
What gets plated in electroplating. The metal that is the cathode gets coated during electroplating. Zinc plating has a thin layer of zinc which only offers a fraction of rust. Copper goes into solution at the anode as it is plated at the cathode. Phosphating is the process of converting a steel surface to iron phosphate. When a metal object is electroplated with gold it is called gold plating.
When a metal object is electroplated with gold it is called gold plating.
Why anode gets thinner after electroplating. The metal that is the cathode gets coated during electroplating. What gets plated in electroplating. The zinc plating occurs through an electric current. What metals gets coated during electroplating. For example copper and steels can be electroplated.
 Source: byjus.com
Source: byjus.com
A kinds of metals can be electroplated such as gold silver tin zinc copper cadmium. A kinds of metals can be electroplated such as gold silver tin zinc copper cadmium. Phosphating is the process of converting a steel surface to iron phosphate. Electroplating is the process of depositing a metal coating onto the surface of an object through the use of an electrical current. Electroplating is widely used in indus.
 Source: en.wikipedia.org
Source: en.wikipedia.org
When a metal object is electroplated with nickel it is called nickel plating. What metals gets coated during electroplating. Zinc plating has a thin layer of zinc which only offers a fraction of rust. Phosphating is the process of converting a steel surface to iron phosphate. Why anode gets thinner after electroplating.
 Source: en.wikipedia.org
Source: en.wikipedia.org
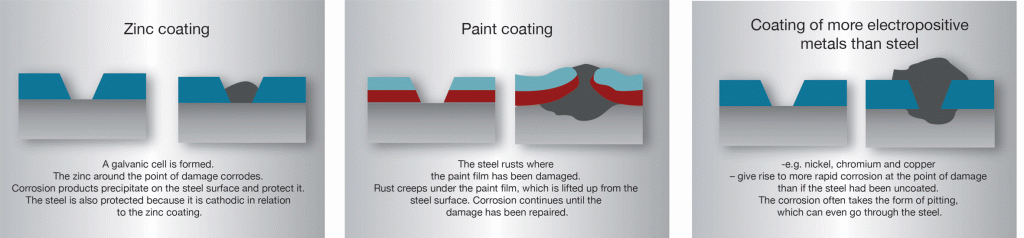
The part to be coated acts as the cathode of an electrolytic cell. Zinc plating has a thin layer of zinc which only offers a fraction of rust. The zinc plating occurs through an electric current. Electroplating has evolved into a highly complex process requiring a high level of precision and expertise. Phosphating is the process of converting a steel surface to iron phosphate.
 Source: britannica.com
Source: britannica.com
And the anode is usually either a block of that metal or of some inert conductive material. Zinc plating has a thin layer of zinc which only offers a fraction of rust. What metals gets coated during electroplating. When a metal object is electroplated with copper it is called copper plating. A kinds of metals can be electroplated such as gold silver tin zinc copper cadmium.
 Source: bifarmafranquias.com.br
Source: bifarmafranquias.com.br
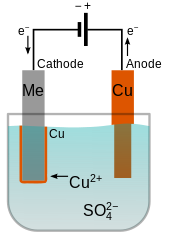
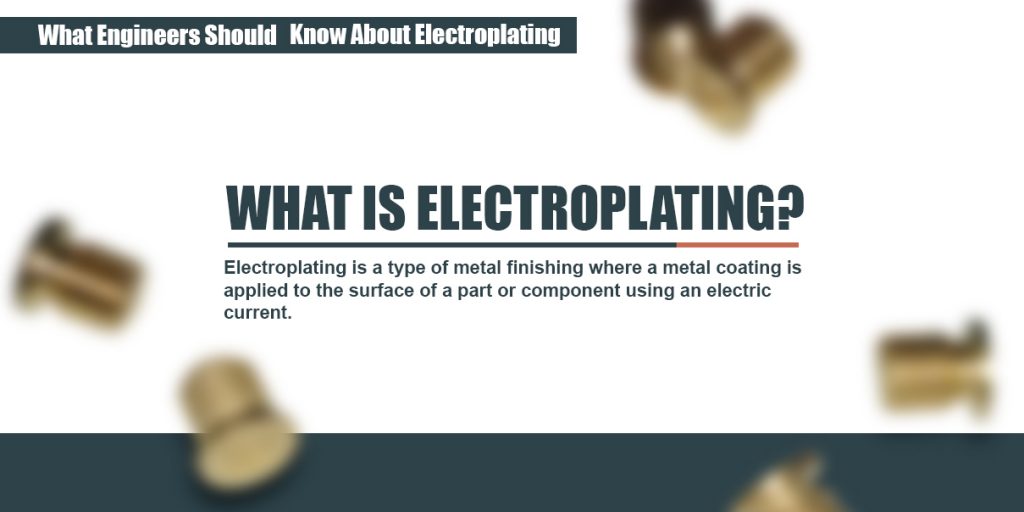
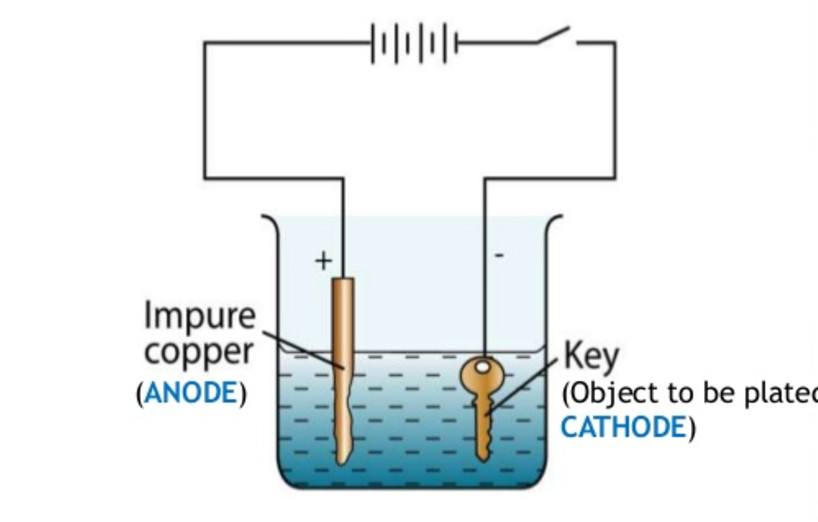
Zinc plating has a thin layer of zinc which only offers a fraction of rust. The cathode gets coated during electroplating. The part to be coated acts as the cathode of an electrolytic cell. What gets plated in electroplating. A simple example of the electroplating process is the electroplating of copper in which the metal to be plated copper is used as the anode and the electrolyte solution contains the ion of the metal to be plated cu 2 in this example.
 Source: nordicgalvanizers.com
Source: nordicgalvanizers.com
For example copper and steels can be electroplated. The metal that is the cathode gets coated during electroplating. The metal that is the cathode gets coated during electroplating. A kinds of metals can be electroplated such as gold silver tin zinc copper cadmium. A simple example of the electroplating process is the electroplating of copper in which the metal to be plated copper is used as the anode and the electrolyte solution contains the ion of the metal to be plated cu 2 in this example.
 Source: brainly.com
Source: brainly.com
What metals gets coated during electroplating. The part to be coated acts as the cathode of an electrolytic cell. What gets plated in electroplating. Phosphating is the process of converting a steel surface to iron phosphate. Why anode gets thinner after electroplating.
 Source: bifarmafranquias.com.br
Source: bifarmafranquias.com.br
What gets plated in electroplating. The zinc plating occurs through an electric current. For example copper and steels can be electroplated. A kinds of metals can be electroplated such as gold silver tin zinc copper cadmium. The part to be coated acts as the cathode of an electrolytic cell.
 Source: bifarmafranquias.com.br
Source: bifarmafranquias.com.br
When a metal object is electroplated with copper it is called copper plating. What metals gets coated during electroplating. Why anode gets thinner after electroplating. The zinc plating occurs through an electric current. Electroplating is the process of depositing a metal coating onto the surface of an object through the use of an electrical current.
 Source: classnotes.org.in
Source: classnotes.org.in
Phosphating is the process of converting a steel surface to iron phosphate. When metal object is electroplated with silver it is called silver plating. While both the methods involve coating the metal with zinc the main difference occurs in the thickness of the coating. The cathode gets coated during electroplating. Phosphating is the process of converting a steel surface to iron phosphate.
 Source: bifarmafranquias.com.br
Source: bifarmafranquias.com.br
Why anode gets thinner after electroplating. When a metal object is electroplated with gold it is called gold plating. The metal that is the cathode gets coated during electroplating. What metals gets coated during electroplating. Why anode gets thinner after electroplating.
 Source: sharrettsplating.com
Source: sharrettsplating.com
For example copper and steels can be electroplated. From afar these methods might look pretty much the same but the reality is pretty different. Electroplating is widely used in indus. When a metal object is electroplated with gold it is called gold plating. Electroplating is the process of depositing a metal coating onto the surface of an object through the use of an electrical current.
 Source: bifarmafranquias.com.br
Source: bifarmafranquias.com.br
When a metal object is electroplated with nickel it is called nickel plating. The metal that is the cathode gets coated during electroplating. The part to be coated acts as the cathode of an electrolytic cell. For example copper and steels can be electroplated. The electrolyte is a solution of a salt of the metal to be coated.
 Source: bifarmafranquias.com.br
Source: bifarmafranquias.com.br
The metal that is the cathode gets coated during electroplating. What metals gets coated during electroplating. Phosphating is the process of converting a steel surface to iron phosphate. The current is provided by an external power supply. When a metal object is electroplated with copper it is called copper plating.
 Source: bifarmafranquias.com.br
Source: bifarmafranquias.com.br
A kinds of metals can be electroplated such as gold silver tin zinc copper cadmium. Why anode gets thinner after electroplating. A kinds of metals can be electroplated such as gold silver tin zinc copper cadmium. From afar these methods might look pretty much the same but the reality is pretty different. For example copper and steels can be electroplated.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title which metal gets coated during electroplating by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





