What type of ions do bases release
What Type Of Ions Do Bases Release. Citric acid lactic acid tartaric acid and acetic acid. These substances release hydroxide ions oh ions when dissolved in water. Example naoh h20 na oh what type of substance provides. H3o 1 is the.
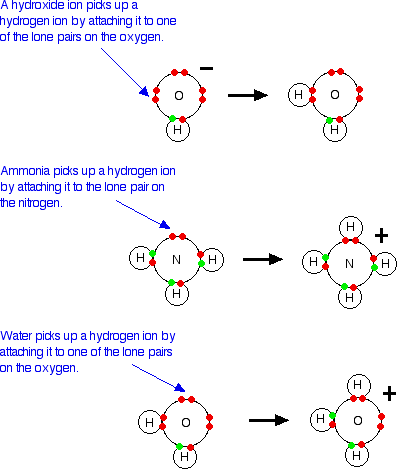 Theories Of Acids And Bases From chemguide.co.uk
Theories Of Acids And Bases From chemguide.co.uk
These substances release hydroxide ions oh ions when dissolved in water. Strong bases hydrolyze in water almost completely resulting in the leveling effect in this process the water molecule combines with a strong base due to the water s amphoteric ability. When you combine an acid and a base the products are a salt and water. The ph values corresponding to bases are always greater than 7. A solution with a high number of hydroxide ions is basic and has a high ph value. And a hydroxide ion is released.
A solution with a high number of hydroxide ions is basic and has a high ph value.
Bases are bitter tasting substances which have the ability to turn red litmus paper blue. The most common example is sodium hydroxide naoh. Not every base contains hydroxide ions for example does not. What kinds of ions do bases release. These substances release hydroxide ions oh ions when dissolved in water. The hydroxide ions can combine with hydrogen ions therefore decreasing the number of hydrogen ions in the solution to form more water.
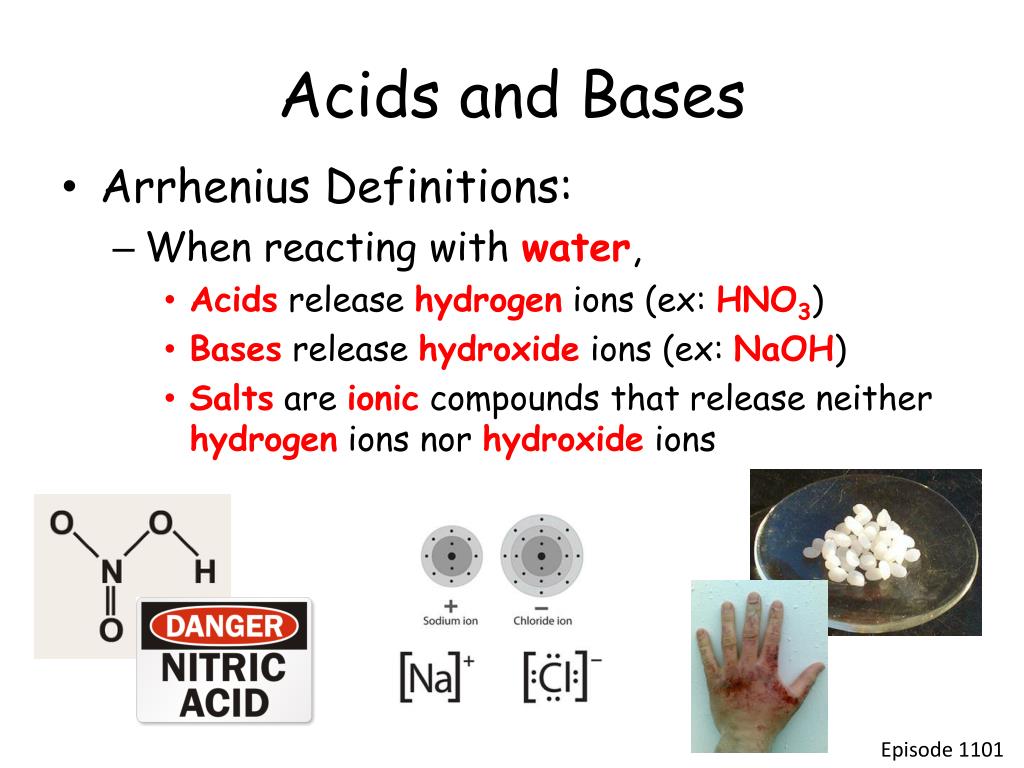 Source: slideserve.com
Source: slideserve.com
H h 2o h 3o. Bases produce hydroxide ions oh. Buffers ph acids and bases. Bases are bitter tasting substances which have the ability to turn red litmus paper blue. Strong bases hydrolyze in water almost completely resulting in the leveling effect in this process the water molecule combines with a strong base due to the water s amphoteric ability.
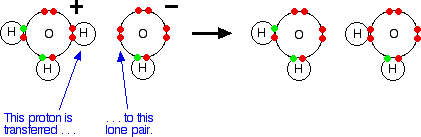 Source: chemguide.co.uk
Source: chemguide.co.uk
Hcl h2o h3o 1 cl 1. In their aqueous solutions bases act as good conductors of electricity. The ph values corresponding to bases are always greater than 7. Strong bases hydrolyze in water almost completely resulting in the leveling effect in this process the water molecule combines with a strong base due to the water s amphoteric ability. Oh a reaction between an acid and base is called.
 Source: dronstudy.com
Source: dronstudy.com
H h 2o h 3o. The answer is that bases release in water is hydroxide ions. A solution with a high number of hydrogen ions is acidic and has a low ph value. The most common example is sodium hydroxide naoh. Name four organic acids.
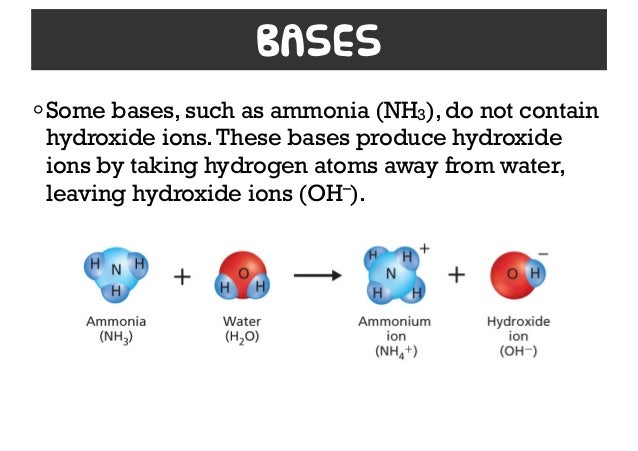 Source: slideshare.net
Source: slideshare.net
Neutralisation is a chemical reaction that occurs when you mix an acid and a base together. The base cancels out the. When an acid is dissolved in water the concentration of the hydronium ions increases. Oh a reaction between an acid and base is called. Buffers ph acids and bases.
 Source: chemguide.co.uk
Source: chemguide.co.uk
In their aqueous solutions bases act as good conductors of electricity. Hcl h2o h3o 1 cl 1. A solution with a high number of hydrogen ions is acidic and has a low ph value. What kinds of ions do bases release. Any type of base that has one or more oh ions in it and is soluble in water will release hydroxide ions in aqueous solution.
 Source: slideplayer.com
Source: slideplayer.com
A hydrogen ion is a bare proton that associates with a water molecule so the h ions produced by an acid exist as h3o ions. Neutralisation is a chemical reaction that occurs when you mix an acid and a base together. And a hydroxide ion is released. A is any ionic compound that can be made from the neutralization of an acid with a base. Hcl h2o h3o 1 cl 1.
 Source: slideshare.net
Source: slideshare.net
Bases are molecules that can split apart in water and release hydroxide ions. Neutralisation is a chemical reaction that occurs when you mix an acid and a base together. Hcl h2o h3o 1 cl 1. Bases produce hydroxide ions oh. And a hydroxide ion is released.
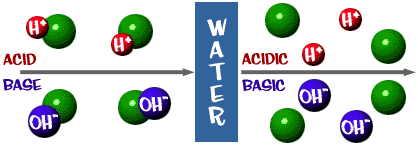 Source: chem4kids.com
Source: chem4kids.com
Strong bases hydrolyze in water almost completely resulting in the leveling effect in this process the water molecule combines with a strong base due to the water s amphoteric ability. Bases are bitter tasting substances which have the ability to turn red litmus paper blue. When you combine an acid and a base the products are a salt and water. The ph values corresponding to bases are always greater than 7. In their aqueous solutions bases act as good conductors of electricity.
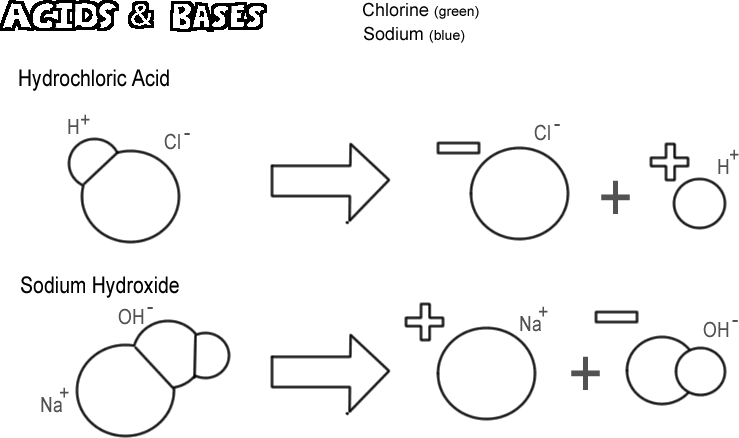 Source: biologycorner.com
Source: biologycorner.com
A solution with a high number of hydroxide ions is basic and has a high ph value. What kinds of ions do bases release. The most common example is sodium hydroxide naoh. Hcl h2o h3o 1 cl 1. In their aqueous solutions bases act as good conductors of electricity.
 Source: slideplayer.com
Source: slideplayer.com
H3o 1 is the. Name four organic acids. Bases are molecules that can split apart in water and release hydroxide ions. Hydrochloric acid hcl. These substances release hydroxide ions oh ions when dissolved in water.
 Source: slideplayer.com
Source: slideplayer.com
When base is dissolved in water the concentration of the hydroxide ions increases. Citric acid lactic acid tartaric acid and acetic acid. Oh a reaction between an acid and base is called. A solution with a high number of hydroxide ions is basic and has a high ph value. Not every base contains hydroxide ions for example does not.
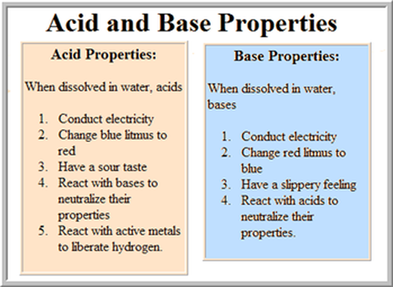 Source: sciencewithmrsb.weebly.com
Source: sciencewithmrsb.weebly.com
Very strong bases can even deprotonate very weakly acidic c h groups in the absence of water. When naoh is added to water it splits apart into na and oh. A solution with a high number of hydroxide ions is basic and has a high ph value. When base is dissolved in water the concentration of the hydroxide ions increases. The ph values corresponding to bases are always greater than 7.
 Source: www2.nau.edu
Source: www2.nau.edu
Buffers ph acids and bases. Example naoh h20 na oh what type of substance provides. The hydroxide ions can combine with hydrogen ions therefore decreasing the number of hydrogen ions in the solution to form more water. These substances release hydroxide ions oh ions when dissolved in water. A hydrogen ion is a bare proton that associates with a water molecule so the h ions produced by an acid exist as h3o ions.
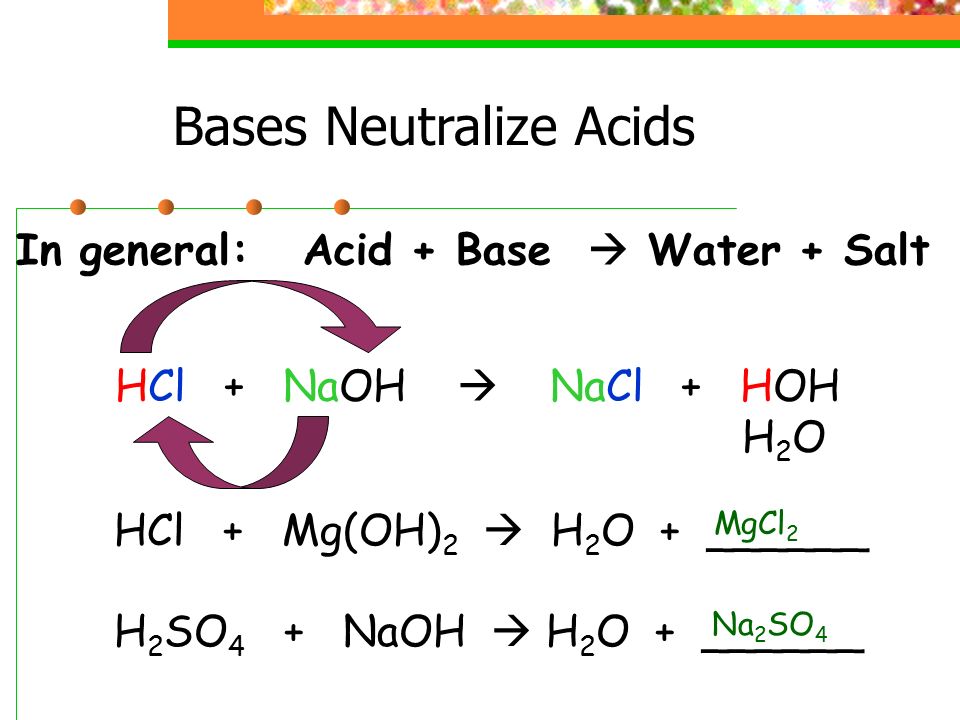 Source: sciencewithmrsb.weebly.com
Source: sciencewithmrsb.weebly.com
Hydrochloric acid hcl. Bases are molecules that can split apart in water and release hydroxide ions. When naoh is added to water it splits apart into na and oh. The ph of a solution is a measure of the concentration of hydrogen ions in the solution. Neutralisation is a chemical reaction that occurs when you mix an acid and a base together.
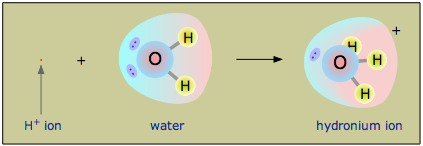 Source: chem.libretexts.org
Source: chem.libretexts.org
Oh a reaction between an acid and base is called. Let me show you some examples. H3o 1 is the. A solution with a high number of hydrogen ions is acidic and has a low ph value. Any type of base that has one or more oh ions in it and is soluble in water will release hydroxide ions in aqueous solution.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what type of ions do bases release by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






