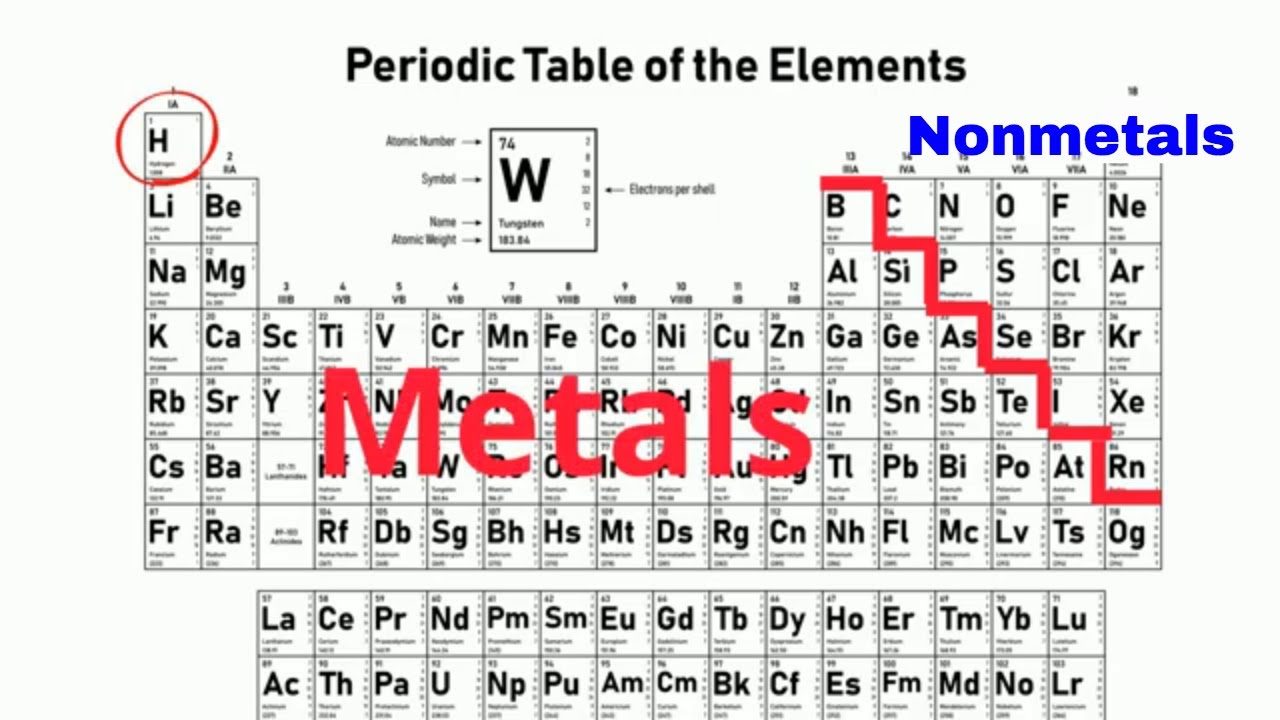
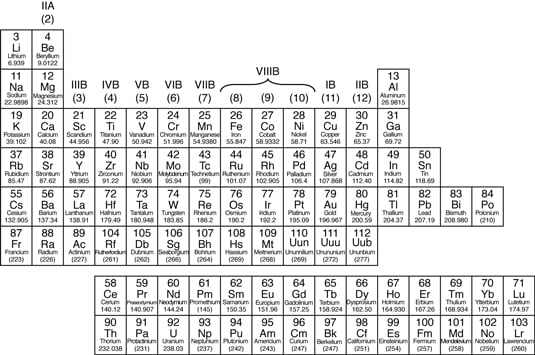
What properties identify nonmetals
What Properties Identify Nonmetals. Are brittle when solid. It is a good conductor of electricity and is used in making electrodes. Solid nonmetals are generally brittle with little or no metallic luster. Typical nonmetals have a dull coloured or colourless appearance.
 Nonmetal Wikipedia From en.wikipedia.org
Nonmetal Wikipedia From en.wikipedia.org
Solid nonmetals are generally brittle with little or no metallic luster. Carbon graphite is an exception. Non metals are bad conductors of heat and electricity non metals are generally bad conductors of heat and electricity. Some physical properties of non metals are listed below. Properties of nonmetals. Most nonmetals have the ability to gain electrons easily.
Poor conductors of electricity and heat.
Non metals are bad conductors of heat and electricity non metals are generally bad conductors of heat and electricity. Properties of nonmetals. And have acidic oxides. Solid nonmetals are generally brittle with little or no metallic luster. Nonmetals display a wide range of chemical properties and reactivities. Carbon graphite is an exception.
 Source: studylib.net
Source: studylib.net
Non metals are bad conductors of heat and electricity non metals are generally bad conductors of heat and electricity. Maybe solids liquids or gases at room temperature. Nonmetals display a wide range of chemical properties and reactivities. Carbon graphite is an exception. Properties of nonmetals.
 Source: pinterest.com
Source: pinterest.com
A few elements have properties that are either anomalous given their category or otherwise extraordinary. They are generally poor conductors of heat and electricity. Carbon graphite is an exception. Some physical properties of non metals are listed below. These are not sonorous.
 Source: youtube.com
Source: youtube.com
Properties of nonmetals. Non metals are bad conductors of heat and electricity non metals are generally bad conductors of heat and electricity. Nonmetals display a wide range of chemical properties and reactivities. Solid nonmetals are generally brittle with little or no metallic luster. Maybe solids liquids or gases at room temperature.
 Source: slideplayer.com
Source: slideplayer.com
Carbon graphite is an exception. Typical nonmetals have a dull coloured or colourless appearance. It is a good conductor of electricity and is used in making electrodes. Chemical properties of non metals. Properties of non metals physical properties of non metals.
 Source: thoughtco.com
Source: thoughtco.com
Nonmetals display a wide range of chemical properties and reactivities. Properties of non metals physical properties of non metals. Are poor conductors of heat and electricity. Typical nonmetals have a dull coloured or colourless appearance. Carbon graphite is an exception.
 Source: sciencenotes.org
Source: sciencenotes.org
They are generally poor conductors of heat and electricity. Poor conductors of electricity and heat. Most nonmetals have the ability to gain electrons easily. Chemical properties of non metals. It is a good conductor of electricity and is used in making electrodes.
 Source: slideshare.net
Source: slideshare.net
Non metals are bad conductors of heat and electricity non metals are generally bad conductors of heat and electricity. Nonmetals display a wide range of chemical properties and reactivities. Some physical properties of non metals are listed below. Nonmetals have high ionization energies and electronegativities. Are brittle when solid.
 Source: quora.com
Source: quora.com
Are poor conductors of heat and electricity. Typical nonmetals have a dull coloured or colourless appearance. Properties of non metals physical properties of non metals. It is a good conductor of electricity and is used in making electrodes. Are brittle when solid.
 Source: sciencenotes.org
Source: sciencenotes.org
Nonmetals have high ionization energies and electronegativities. And have acidic oxides. Some physical properties of non metals are listed below. A few elements have properties that are either anomalous given their category or otherwise extraordinary. They are generally poor conductors of heat and electricity.
 Source: pinterest.com
Source: pinterest.com
Some physical properties of non metals are listed below. Nonmetals display a wide range of chemical properties and reactivities. Are brittle when solid. And have acidic oxides. These are not sonorous.

And have acidic oxides. Solid nonmetals are generally brittle with little or no metallic luster. Carbon graphite is an exception. They are generally poor conductors of heat and electricity. Properties of non metals physical properties of non metals.

These are not sonorous. Poor conductors of electricity and heat. They are generally poor conductors of heat and electricity. Properties of non metals physical properties of non metals. Most nonmetals have the ability to gain electrons easily.
 Source: slideplayer.com
Source: slideplayer.com
Solid nonmetals are generally brittle with little or no metallic luster. A few elements have properties that are either anomalous given their category or otherwise extraordinary. It is a good conductor of electricity and is used in making electrodes. Carbon graphite is an exception. Nonmetals have high ionization energies and electronegativities.
 Source: dummies.com
Source: dummies.com
Nonmetals have high ionization energies and electronegativities. Chemical properties of non metals. Properties of non metals physical properties of non metals. Non metals are bad conductors of heat and electricity non metals are generally bad conductors of heat and electricity. They are generally poor conductors of heat and electricity.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Nonmetals have high ionization energies and electronegativities. Are brittle when solid. Are poor conductors of heat and electricity. They are generally poor conductors of heat and electricity. Solid nonmetals are generally brittle with little or no metallic luster.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what properties identify nonmetals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.