What makes metals malleable and ductile
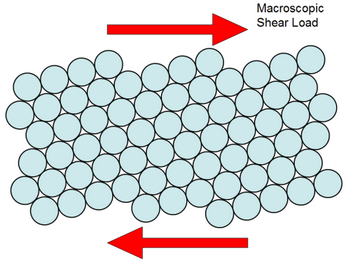
What Makes Metals Malleable And Ductile. In metallurgy a malleable material is one that can be easily formed by hammering rolling or pressing it. This is because metals have free negative electrons that surround positive metal ions. In a metallic piece the atoms are identical and they are piled up like layers of beads in the bottom of a flat box each bead touching its neighbors. They exist as individual atoms.
 Why Are Metals Malleable Socratic From socratic.org
Why Are Metals Malleable Socratic From socratic.org
Gold is the most malleable and ductile of all known metals. Metals are malleable and ductile because they are made of hexagonal and cubic packed structures that can be moved by applying force to them. The sliding of atoms when force is applied is the reason that metals can change their shapes. In ductile iron the materials added during the melt help form more regular spheres of carbon. They are also ductile which means they can be easily drawn into wires. The material is considered malleable because it can be manipulated under compressive stress.
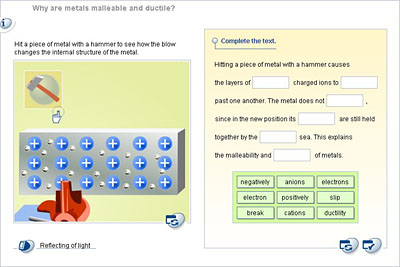
This is because metals have free negative electrons that surround positive metal ions.
Thin sheets of gold known as gold leaf are primarily used in arts and crafts for gilding. The sliding of atoms when force is applied is the reason that metals can change their shapes. By contrast ductility is the ability of a material to be manipulated under tensile stress which is how easily it can be stretched. The material is considered malleable because it can be manipulated under compressive stress. They exist as individual atoms. When pressure is applied these electrons ensure that the atoms stay glued together making the material both malleable and ductile without breaking apart or becoming brittle.
 Source: friedbiochem.weebly.com
Source: friedbiochem.weebly.com
This spheroid composition gives the metal fewer internal flaws making it stronger and allowing it to be bent twisted and elongated more successfully than malleable iron. They exist as individual atoms. The metals are malleable because they don t make molecules. A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side. The sliding of atoms when force is applied is the reason that metals can change their shapes.
 Source: e-education.psu.edu
Source: e-education.psu.edu
When force is applied the atoms slide from one plane past atoms in a different plane. This spheroid composition gives the metal fewer internal flaws making it stronger and allowing it to be bent twisted and elongated more successfully than malleable iron. A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side. When force is applied the atoms slide from one plane past atoms in a different plane. Metals are malleable and ductile because they are made of hexagonal and cubic packed structures that can be moved by applying force to them.
 Source: slideplayer.com
Source: slideplayer.com
This spheroid composition gives the metal fewer internal flaws making it stronger and allowing it to be bent twisted and elongated more successfully than malleable iron. The sliding of atoms when force is applied is the reason that metals can change their shapes. By contrast ductility is the ability of a material to be manipulated under tensile stress which is how easily it can be stretched. When force is applied the atoms slide from one plane past atoms in a different plane. A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side.
 Source: quora.com
Source: quora.com
They are also ductile which means they can be easily drawn into wires. They give the metal more strength and flexibility than earlier cast irons. In ductile iron the materials added during the melt help form more regular spheres of carbon. When force is applied the atoms slide from one plane past atoms in a different plane. Metals are malleable meaning that they can be formed into other shapes such as thin sheets or foils without breaking or cracking.
 Source: eurekaelearning.com
Source: eurekaelearning.com
The metals are malleable because they don t make molecules. In ductile iron the materials added during the melt help form more regular spheres of carbon. When force is applied the atoms slide from one plane past atoms in a different plane. Gold is the most malleable and ductile of all known metals. The sliding of atoms when force is applied is the reason that metals can change their shapes.
 Source: mytutorialworld.com
Source: mytutorialworld.com
Thin sheets of gold known as gold leaf are primarily used in arts and crafts for gilding. This is because metals have free negative electrons that surround positive metal ions. Thin sheets of gold known as gold leaf are primarily used in arts and crafts for gilding. Metals are malleable and ductile because they are made of hexagonal and cubic packed structures that can be moved by applying force to them. They give the metal more strength and flexibility than earlier cast irons.
 Source: inchemistry.acs.org
Source: inchemistry.acs.org
They give the metal more strength and flexibility than earlier cast irons. Thin sheets of gold known as gold leaf are primarily used in arts and crafts for gilding. They give the metal more strength and flexibility than earlier cast irons. A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side. They exist as individual atoms.
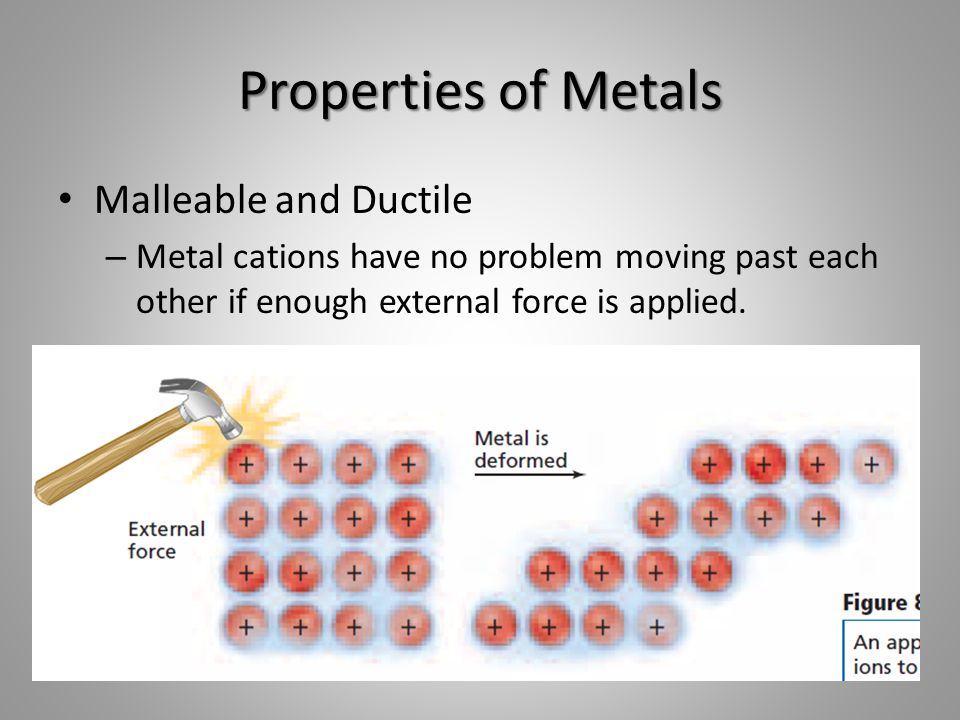 Source: slideplayer.com
Source: slideplayer.com
In metallurgy a malleable material is one that can be easily formed by hammering rolling or pressing it. Gold is the most malleable and ductile of all known metals. This spheroid composition gives the metal fewer internal flaws making it stronger and allowing it to be bent twisted and elongated more successfully than malleable iron. The material is considered malleable because it can be manipulated under compressive stress. Gold is the most malleable and ductile of all known metals.
 Source: blog.melscience.com
Source: blog.melscience.com
A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side. By contrast ductility is the ability of a material to be manipulated under tensile stress which is how easily it can be stretched. They are also ductile which means they can be easily drawn into wires. This spheroid composition gives the metal fewer internal flaws making it stronger and allowing it to be bent twisted and elongated more successfully than malleable iron. In ductile iron the materials added during the melt help form more regular spheres of carbon.
 Source: quora.com
Source: quora.com
Metals are malleable meaning that they can be formed into other shapes such as thin sheets or foils without breaking or cracking. A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side. By contrast ductility is the ability of a material to be manipulated under tensile stress which is how easily it can be stretched. In a metallic piece the atoms are identical and they are piled up like layers of beads in the bottom of a flat box each bead touching its neighbors. A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side.
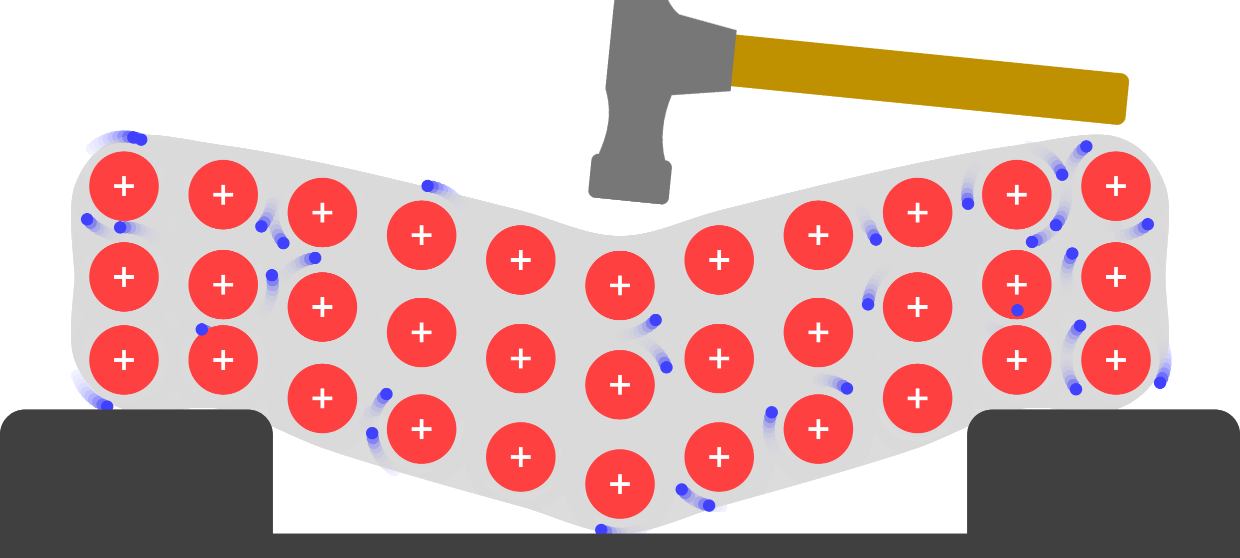 Source: javalab.org
Source: javalab.org
When pressure is applied these electrons ensure that the atoms stay glued together making the material both malleable and ductile without breaking apart or becoming brittle. They exist as individual atoms. When pressure is applied these electrons ensure that the atoms stay glued together making the material both malleable and ductile without breaking apart or becoming brittle. When force is applied the atoms slide from one plane past atoms in a different plane. In a metallic piece the atoms are identical and they are piled up like layers of beads in the bottom of a flat box each bead touching its neighbors.
Source: chemguide.co.uk
A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side. In a metallic piece the atoms are identical and they are piled up like layers of beads in the bottom of a flat box each bead touching its neighbors. When force is applied the atoms slide from one plane past atoms in a different plane. They give the metal more strength and flexibility than earlier cast irons. In metallurgy a malleable material is one that can be easily formed by hammering rolling or pressing it.
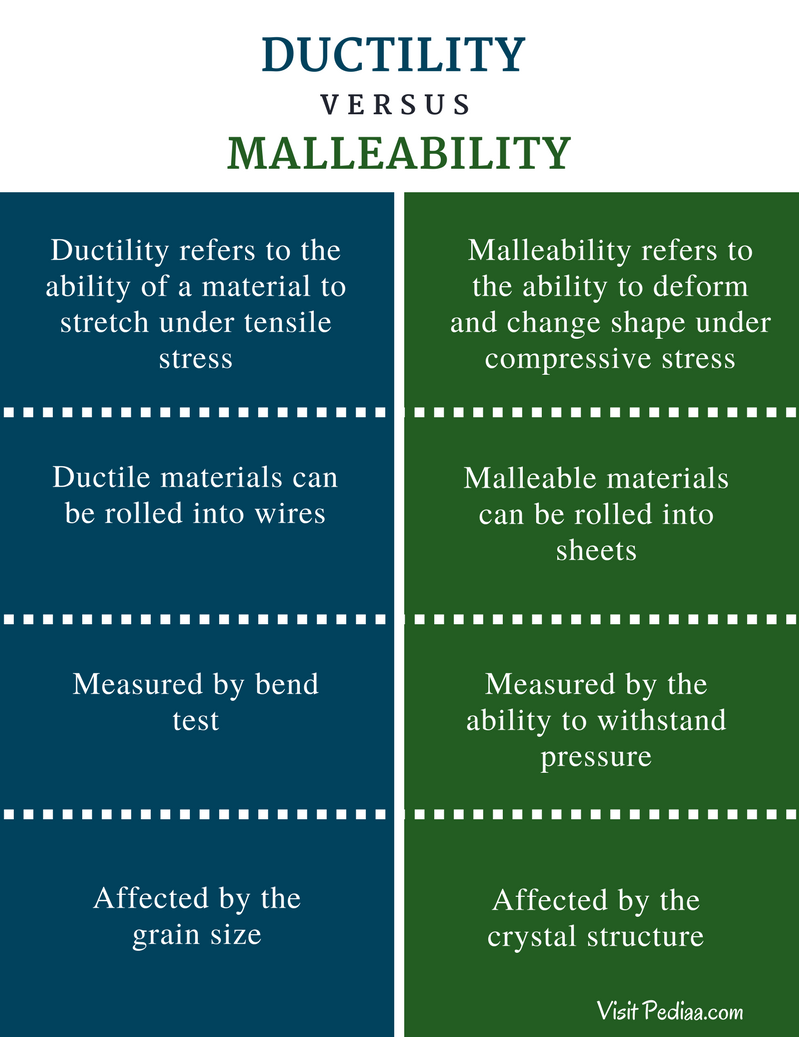 Source: pediaa.com
Source: pediaa.com
Metals are malleable meaning that they can be formed into other shapes such as thin sheets or foils without breaking or cracking. They are also ductile which means they can be easily drawn into wires. When pressure is applied these electrons ensure that the atoms stay glued together making the material both malleable and ductile without breaking apart or becoming brittle. Gold is the most malleable and ductile of all known metals. In metallurgy a malleable material is one that can be easily formed by hammering rolling or pressing it.
 Source: youtube.com
Source: youtube.com
Thin sheets of gold known as gold leaf are primarily used in arts and crafts for gilding. A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side. By contrast ductility is the ability of a material to be manipulated under tensile stress which is how easily it can be stretched. They give the metal more strength and flexibility than earlier cast irons. The sliding of atoms when force is applied is the reason that metals can change their shapes.
 Source: socratic.org
Source: socratic.org
Gold is the most malleable and ductile of all known metals. Metals are malleable meaning that they can be formed into other shapes such as thin sheets or foils without breaking or cracking. A single ounce of gold can be beaten into a sheet measuring roughly 5 meters on a side. They exist as individual atoms. Gold is the most malleable and ductile of all known metals.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what makes metals malleable and ductile by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.