What kind of reaction is used for electroplating
What Kind Of Reaction Is Used For Electroplating. Electroplating is also known simply as plating or as electrodeposition. In electroless or chemical plating the electrons are supplied by a chemical reducing agent in a solution or in the case of immersion plating the substrate itself. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. No external power supply in required.
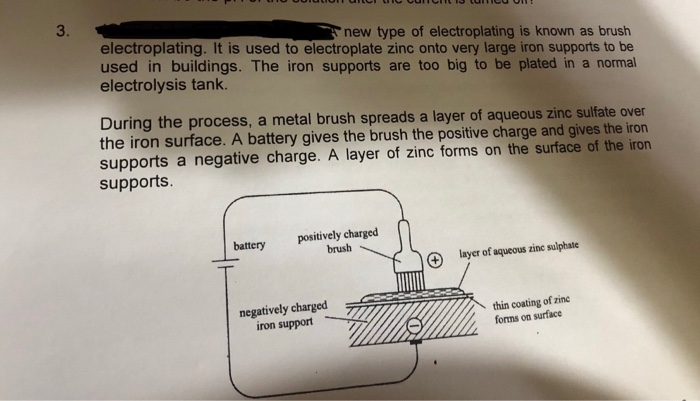 Solved New Type Of Electroplating Is Known As Brush Elect Chegg Com From chegg.com
Solved New Type Of Electroplating Is Known As Brush Elect Chegg Com From chegg.com
Electroplating is a process where a coating of metal is added to a conductor using electricity via a reduction reaction. Iii although copper is a good conductor of electricity it is a non electrolyte. The electrolyte is a solution of a salt of the metal to be coated. Kuntz electroplating was created on 1948 10 16. The electroplating process uses an anode and a cathode. No external power supply in required.
Electroplating is a process where a coating of metal is added to a conductor using electricity via a reduction reaction.
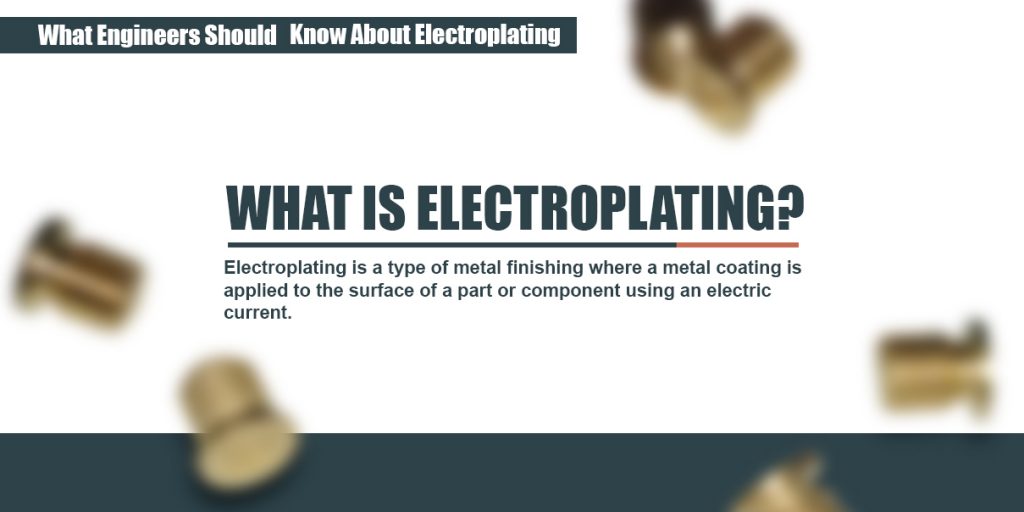
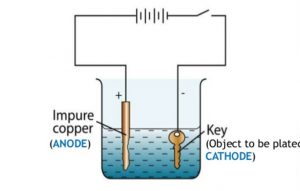
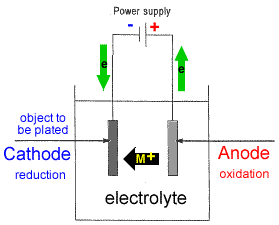
The part to be coated acts as the cathode of an electrolytic cell. When a current is applied to the conductor to be coated metal ions in solution are reduced onto the electrode to form a thin layer. The electroplating process uses an anode and a cathode. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. In electroplating the metal dissolved from the anode can be plated onto the cathode. Copper goes into solution at the anode as it is plated at the cathode.
 Source: sciencedirect.com
Source: sciencedirect.com
Ii in the electroplating of an article with silver the electrolyte sodium argento cyanide solution is preferred over silver nitrate solution. The anode is that where the electrochemical oxidation reaction occurs. I sodium chloride will conduct electricity only in fused or aqueous solution state. In electroplating the metal dissolved from the anode can be plated onto the cathode. And the anode is usually either a block of that metal or of some inert conductive material.
 Source: britannica.com
Source: britannica.com
Iii although copper is a good conductor of electricity it is a non electrolyte. No external power supply in required. Electroplating is widely used in indus. The anode is that where the electrochemical oxidation reaction occurs. I sodium chloride will conduct electricity only in fused or aqueous solution state.
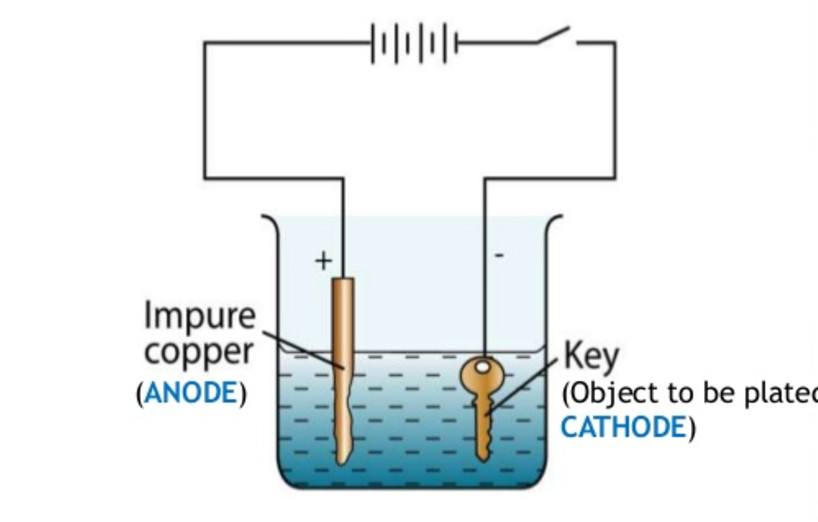 Source: classnotes.org.in
Source: classnotes.org.in
Ii in the electroplating of an article with silver the electrolyte sodium argento cyanide solution is preferred over silver nitrate solution. The chemical reaction used in electroplating is called electrode position electrolysis. Electroplating is widely used in indus. The current is provided by an external power supply. The electrolyte is a solution of a salt of the metal to be coated.
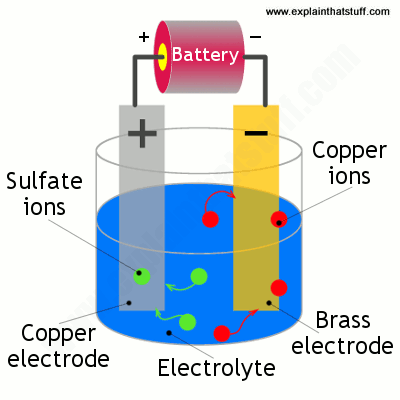 Source: explainthatstuff.com
Source: explainthatstuff.com
The electroplating process uses an anode and a cathode. The part to be coated acts as the cathode of an electrolytic cell. When a current is applied to the conductor to be coated metal ions in solution are reduced onto the electrode to form a thin layer. The electroplating process uses an anode and a cathode. The main type of reaction which takes place in a motor vehicle is the combustion of hydrocarbons.
 Source: byjus.com
Source: byjus.com
The chemical reaction used in electroplating is called electrode position electrolysis. No external power supply in required. Iii although copper is a good conductor of electricity it is a non electrolyte. The chemical reaction used in electroplating is called electrode position electrolysis. I sodium chloride will conduct electricity only in fused or aqueous solution state.
 Source: pinterest.com
Source: pinterest.com
The anode is that where the electrochemical oxidation reaction occurs. I sodium chloride will conduct electricity only in fused or aqueous solution state. When was kuntz electroplating created. Electroless plating is a chemical reaction between metal ions and reducing agents electron source at an active surface. Ii in the electroplating of an article with silver the electrolyte sodium argento cyanide solution is preferred over silver nitrate solution.
 Source: chegg.com
Source: chegg.com
Iii although copper is a good conductor of electricity it is a non electrolyte. Ii in the electroplating of an article with silver the electrolyte sodium argento cyanide solution is preferred over silver nitrate solution. Copper goes into solution at the anode as it is plated at the cathode. Electroplating is a process where a coating of metal is added to a conductor using electricity via a reduction reaction. The chemical reaction used in electroplating is called electrode position electrolysis.
 Source: en.wikipedia.org
Source: en.wikipedia.org
No external power supply in required. In electroplating the metal dissolved from the anode can be plated onto the cathode. When a current is applied to the conductor to be coated metal ions in solution are reduced onto the electrode to form a thin layer. Copper goes into solution at the anode as it is plated at the cathode. The main type of reaction which takes place in a motor vehicle is the combustion of hydrocarbons.
 Source: sharrettsplating.com
Source: sharrettsplating.com
Ii in the electroplating of an article with silver the electrolyte sodium argento cyanide solution is preferred over silver nitrate solution. The chemical reaction used in electroplating is called electrode position electrolysis. In electroplating the metal dissolved from the anode can be plated onto the cathode. The electroplating process uses an anode and a cathode. The main type of reaction which takes place in a motor vehicle is the combustion of hydrocarbons.
 Source: sharrettsplating.com
Source: sharrettsplating.com
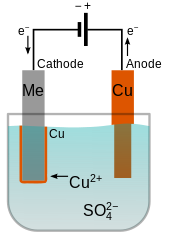
The chemical reaction used in electroplating is called electrode position electrolysis. And the anode is usually either a block of that metal or of some inert conductive material. A simple example of the electroplating process is the electroplating of copper in which the metal to be plated copper is used as the anode and the electrolyte solution contains the ion of the metal to be plated cu 2 in this example. I sodium chloride will conduct electricity only in fused or aqueous solution state. The current is provided by an external power supply.
 Source: classnotes.org.in
Source: classnotes.org.in
Iii although copper is a good conductor of electricity it is a non electrolyte. And the anode is usually either a block of that metal or of some inert conductive material. When a current is applied to the conductor to be coated metal ions in solution are reduced onto the electrode to form a thin layer. When was kuntz electroplating created. Electroplating is also known simply as plating or as electrodeposition.
 Source: ausetute.com.au
Source: ausetute.com.au
In electroless or chemical plating the electrons are supplied by a chemical reducing agent in a solution or in the case of immersion plating the substrate itself. In electroplating the metal dissolved from the anode can be plated onto the cathode. When a current is applied to the conductor to be coated metal ions in solution are reduced onto the electrode to form a thin layer. The part to be coated acts as the cathode of an electrolytic cell. Kuntz electroplating was created on 1948 10 16.
 Source: quora.com
Source: quora.com
When was kuntz electroplating created. Electroplating is a general name for processes that create a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. Copper goes into solution at the anode as it is plated at the cathode. A simple example of the electroplating process is the electroplating of copper in which the metal to be plated copper is used as the anode and the electrolyte solution contains the ion of the metal to be plated cu 2 in this example. The chemical reaction used in electroplating is called electrode position electrolysis.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The main type of reaction which takes place in a motor vehicle is the combustion of hydrocarbons. Ii in the electroplating of an article with silver the electrolyte sodium argento cyanide solution is preferred over silver nitrate solution. Copper goes into solution at the anode as it is plated at the cathode. Electroplating is widely used in indus. A simple example of the electroplating process is the electroplating of copper in which the metal to be plated copper is used as the anode and the electrolyte solution contains the ion of the metal to be plated cu 2 in this example.
 Source: m.youtube.com
Source: m.youtube.com
Kuntz electroplating was created on 1948 10 16. Kuntz electroplating was created on 1948 10 16. The anode is that where the electrochemical oxidation reaction occurs. The main type of reaction which takes place in a motor vehicle is the combustion of hydrocarbons. Electroplating is also known simply as plating or as electrodeposition.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what kind of reaction is used for electroplating by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






