What is the bright line spectrum
What Is The Bright Line Spectrum. The electromagnetic spectrum includes infrared ultraviolet microwaves. Each line represents a unique wavelength and the entire thing is unique to that particular element. Spectral lines are often used to identify atoms and molecules. There are two types of line spectrum.
 Chapter 2 Section 5 From wps.prenhall.com
Chapter 2 Section 5 From wps.prenhall.com
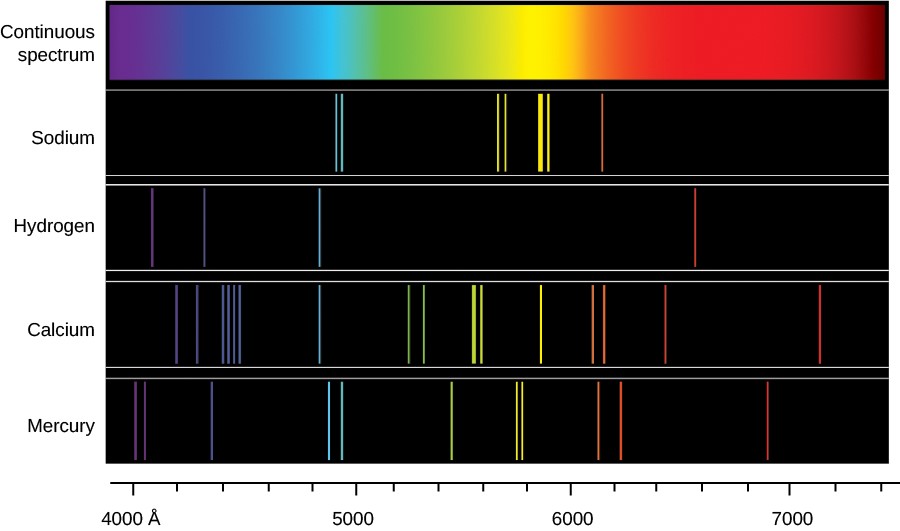
Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies. The first is also called a bright line spectrum and consists of a few brightly colored lines against a dark background. A bright line spectrum is a class of spectra referred to as an emission spectrum. With sodium however we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum at about 589 nm. They are called brightline or emission spectra.
They are called brightline or emission spectra.
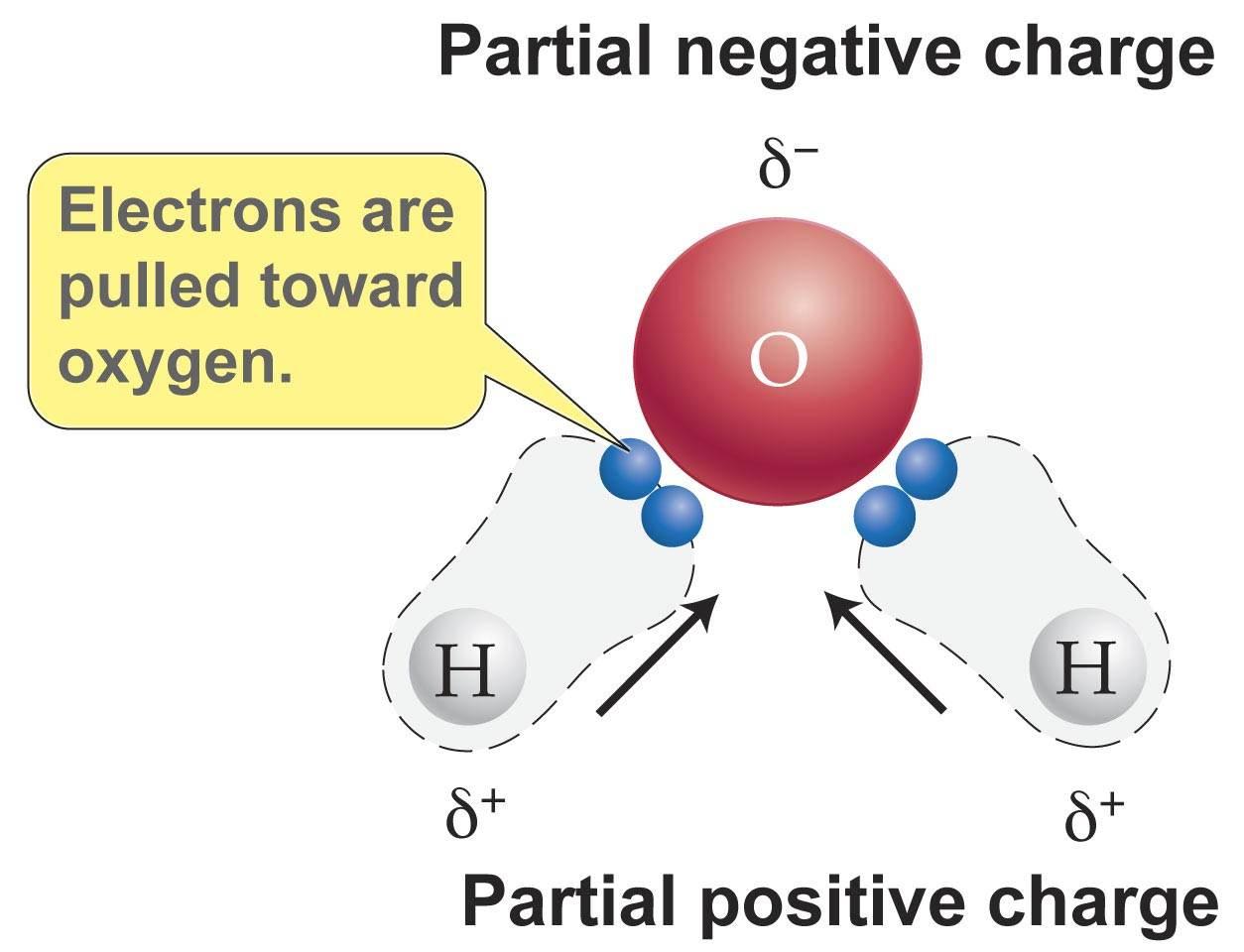
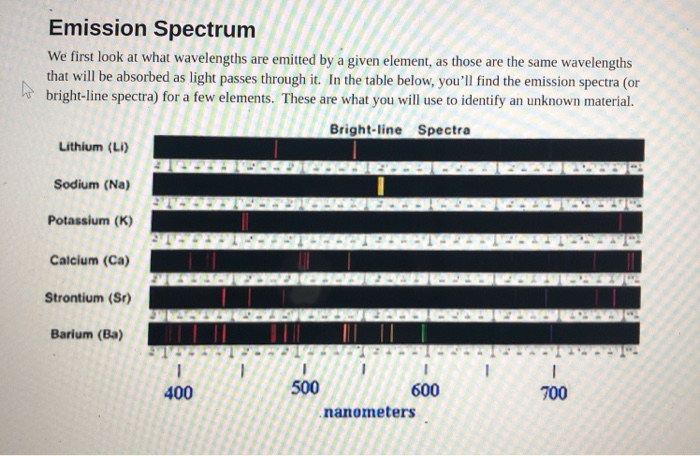
Each line represents a unique wavelength and the entire thing is unique to that particular element. The wavelengths of the lines are characteristic of the element and may form extremely complex patterns. The simplest spectra are those of atomic hydrogen and the alkali atoms e g lithium sodium read more. Electrons of an element particularly valence electrons are in an. Each line represents a unique wavelength and the entire thing is unique to that particular element. Brightline spectrum definition the spectrum of an incandescent substance appearing on a spectrogram as one or more bright lines against a dark background.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
An emission spectrum and an absorption spectrum. The electromagnetic spectrum includes infrared ultraviolet microwaves. The scientific name for light is electromagnetic radiation which includes all wavelengths of light not just visible light. Atoms is known as a line spectrum because the radiation light emitted consists of a series of sharp lines. Spectral lines are often used to identify atoms and molecules.
 Source: youtube.com
Source: youtube.com
A bright line spectrum is a class of spectra referred to as an emission spectrum. Atoms is known as a line spectrum because the radiation light emitted consists of a series of sharp lines. Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. These fingerprints can be compared to the previously collected fingerprints of atoms and molecules and are thus used to identify the atomic and molecular components of stars and planets which would otherwise be impossible. There are two types of line spectrum.
 Source: pa01001042.schoolwires.net
Source: pa01001042.schoolwires.net
The electromagnetic spectrum includes infrared ultraviolet microwaves. Brightline spectrum definition the spectrum of an incandescent substance appearing on a spectrogram as one or more bright lines against a dark background. Atoms is known as a line spectrum because the radiation light emitted consists of a series of sharp lines. These fingerprints can be compared to the previously collected fingerprints of atoms and molecules and are thus used to identify the atomic and molecular components of stars and planets which would otherwise be impossible. Continuous spectra contain no observable gaps.
 Source: chegg.com
Source: chegg.com
The scientific name for light is electromagnetic radiation which includes all wavelengths of light not just visible light. Brightline spectrum definition the spectrum of an incandescent substance appearing on a spectrogram as one or more bright lines against a dark background. With sodium however we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum at about 589 nm. Those incident photons whose energies are exactly equal to the difference between the atom s energy levels are being absorbed. The first is also called a bright line spectrum and consists of a few brightly colored lines against a dark background.
 Source: donklipstein.com
Source: donklipstein.com
Atoms is known as a line spectrum because the radiation light emitted consists of a series of sharp lines. Electrons of an element particularly valence electrons are in an. The simplest spectra are those of atomic hydrogen and the alkali atoms e g lithium sodium read more. An emission spectrum and an absorption spectrum. Atoms is known as a line spectrum because the radiation light emitted consists of a series of sharp lines.
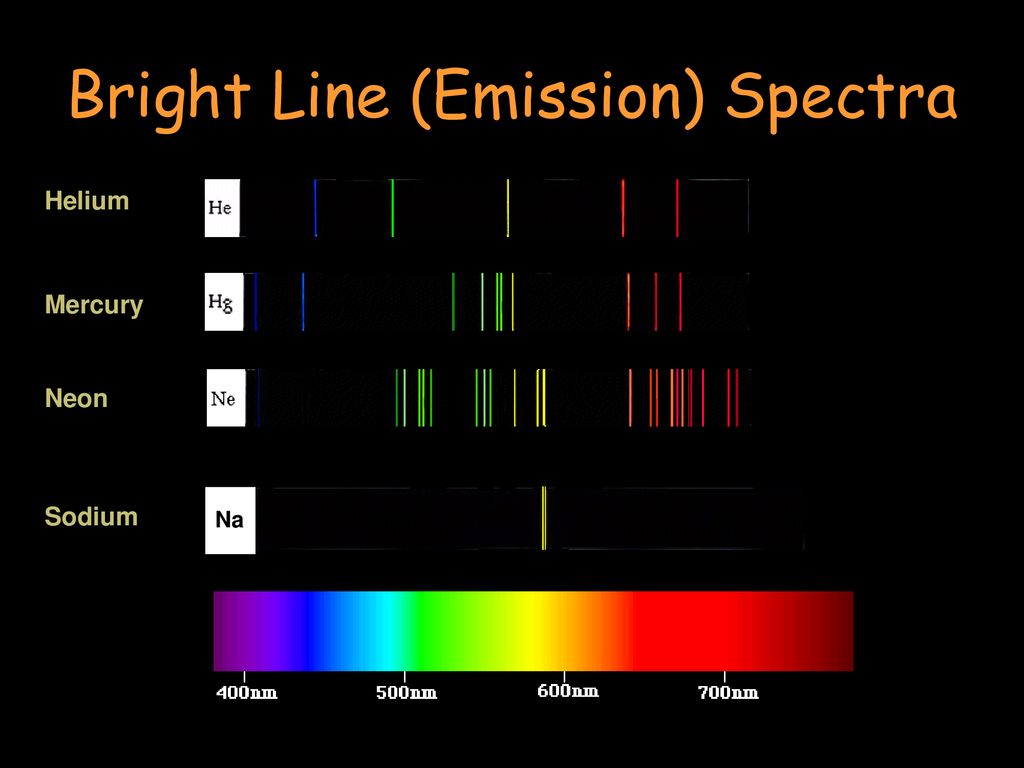 Source: slideplayer.com
Source: slideplayer.com
The first is also called a bright line spectrum and consists of a few brightly colored lines against a dark background. White light is used to excite the atoms. A bright line spectrum is a class of spectra referred to as an emission spectrum. Since the energy levels are discrete only photons of certain frequencies are absorbed. Each line represents a unique wavelength and the entire thing is unique to that particular element.
Source: quora.com
The simplest spectra are those of atomic hydrogen and the alkali atoms e g lithium sodium read more. These lines are emitted when a low pressure gas is put into contact with an electrical discharge. The first is also called a bright line spectrum and consists of a few brightly colored lines against a dark background. A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies. Brightline spectrum definition the spectrum of an incandescent substance appearing on a spectrogram as one or more bright lines against a dark background.
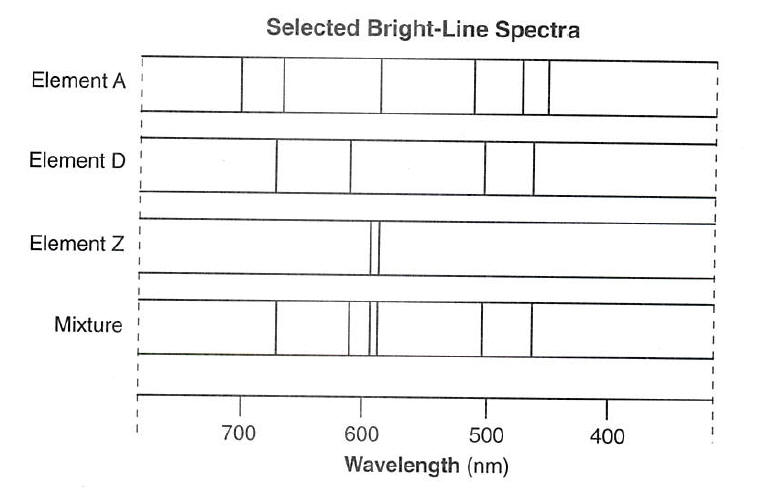 Source: kentchemistry.com
Source: kentchemistry.com
Each line represents a unique wavelength and the entire thing is unique to that particular element. Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. Those incident photons whose energies are exactly equal to the difference between the atom s energy levels are being absorbed. Brightline spectrum definition the spectrum of an incandescent substance appearing on a spectrogram as one or more bright lines against a dark background. An emission spectrum and an absorption spectrum.
 Source: sas.upenn.edu
Source: sas.upenn.edu
The scientific name for light is electromagnetic radiation which includes all wavelengths of light not just visible light. There are huge gaps between lines. Each line represents a unique wavelength and the entire thing is unique to that particular element. Electrons of an element particularly valence electrons are in an. Spectral lines are often used to identify atoms and molecules.
 Source: wps.prenhall.com
Source: wps.prenhall.com
The first is also called a bright line spectrum and consists of a few brightly colored lines against a dark background. White light is used to excite the atoms. The simplest spectra are those of atomic hydrogen and the alkali atoms e g lithium sodium read more. The wavelengths of the lines are characteristic of the element and may form extremely complex patterns. The first is also called a bright line spectrum and consists of a few brightly colored lines against a dark background.
 Source: pinterest.com
Source: pinterest.com
There are huge gaps between lines. Electrons of an element particularly valence electrons are in an. The simplest spectra are those of atomic hydrogen and the alkali atoms e g lithium sodium read more. There are huge gaps between lines. With sodium however we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum at about 589 nm.
 Source: coordinatedscience1.wordpress.com
Source: coordinatedscience1.wordpress.com
Since the energy levels are discrete only photons of certain frequencies are absorbed. The wavelengths of the lines are characteristic of the element and may form extremely complex patterns. Atoms is known as a line spectrum because the radiation light emitted consists of a series of sharp lines. These fingerprints can be compared to the previously collected fingerprints of atoms and molecules and are thus used to identify the atomic and molecular components of stars and planets which would otherwise be impossible. Electrons of an element particularly valence electrons are in an.
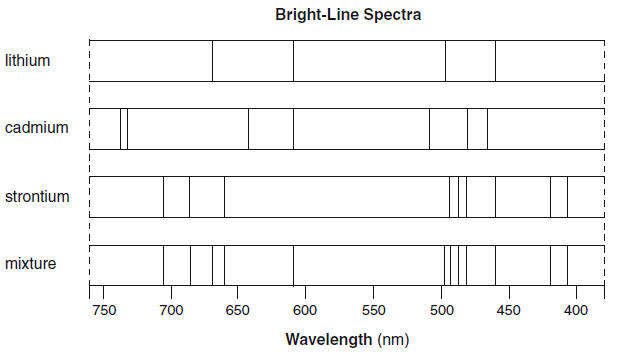 Source: kentchemistry.com
Source: kentchemistry.com
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum resulting from emission or absorption of light in a narrow frequency range compared with the nearby frequencies. Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. With sodium however we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum at about 589 nm. The wavelengths of the lines are characteristic of the element and may form extremely complex patterns. The light emitted by hydrogen atoms is red because of its four characteristic lines the most intense line in its spectrum is in the red portion of the visible spectrum at 656 nm.
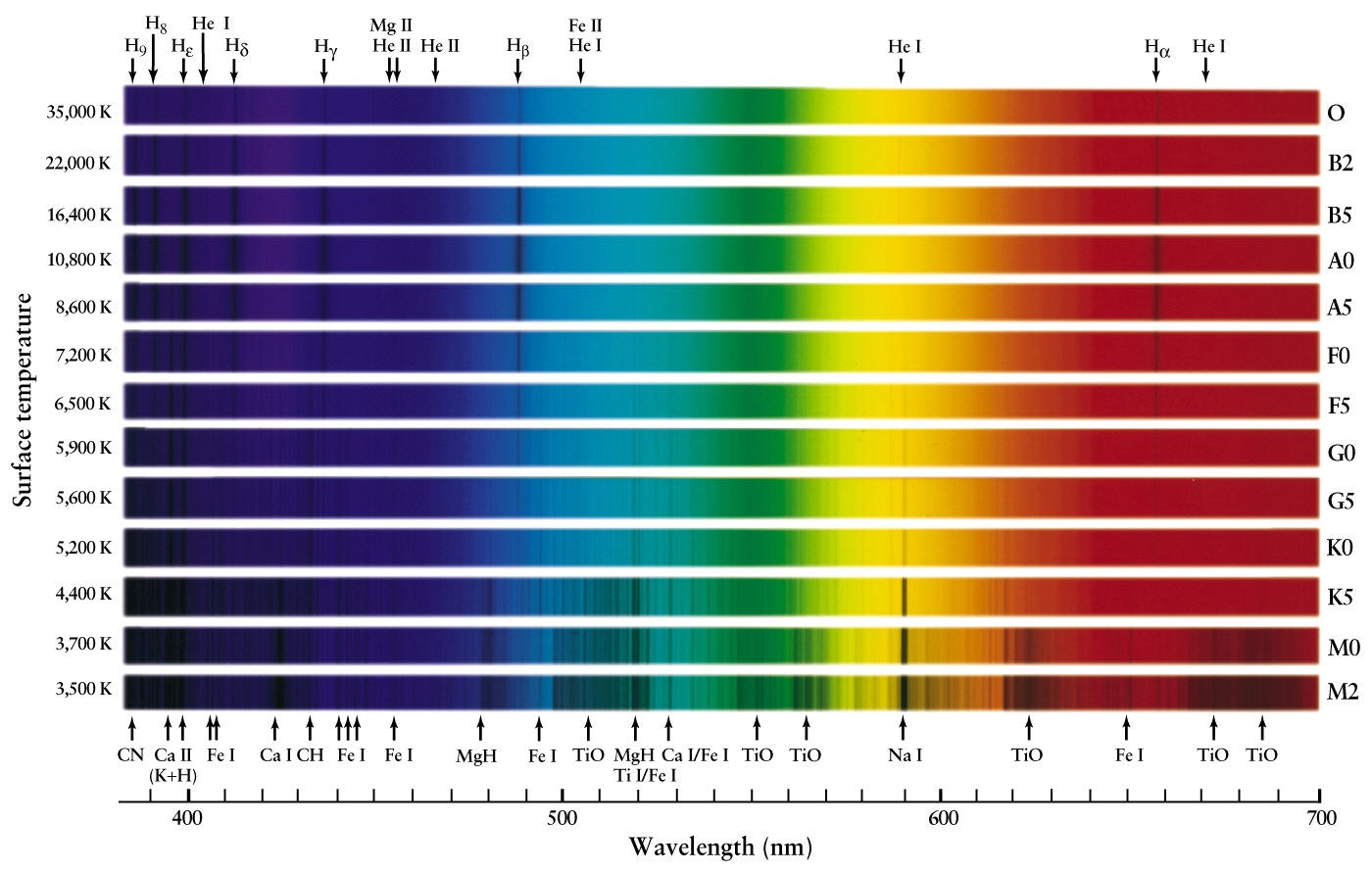 Source: people.highline.edu
Source: people.highline.edu
Electrons of an element particularly valence electrons are in an. Brightline spectrum definition the spectrum of an incandescent substance appearing on a spectrogram as one or more bright lines against a dark background. Since the energy levels are discrete only photons of certain frequencies are absorbed. The first is also called a bright line spectrum and consists of a few brightly colored lines against a dark background. A bright line spectrum is a class of spectra referred to as an emission spectrum.
 Source: simple.wikipedia.org
Source: simple.wikipedia.org
Line spectrum is either an absorption spectrum dark lines in a bright background or an emission spectrum bright lines in the dark background. These lines are emitted when a low pressure gas is put into contact with an electrical discharge. An emission spectrum and an absorption spectrum. There are two types of line spectrum. Spectral lines are often used to identify atoms and molecules.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is the bright line spectrum by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.