What is a voltaic battery
What Is A Voltaic Battery. It consists of two separate half cells. The use of the voltaic cell appears to date back to ancient times with the discovery of the baghdad battery in 1936 a series of galvanic cells placed in large jars from sometime in the first few centuries ad. When a battery is supplying electric power its positive terminal is the cathode. By their nature they produce direct current.
 Color Online A Schematic Illustration Of A Voltaic Pile B Download Scientific Diagram From researchgate.net
Color Online A Schematic Illustration Of A Voltaic Pile B Download Scientific Diagram From researchgate.net
Volta was the inventor of the voltaic pile the first electrical battery. It originated as a schematic drawing of the earliest type of battery a voltaic pile. A voltaic pile is the first type of true electric battery capable of a sustained output of electric current. A betavoltaic device is a type of nuclear battery which generates electric current from beta particles emitted from a radioactive source using semiconductor junctions. The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit. A galvanic cell or voltaic cell named after luigi galvani or alessandro volta respectively is an electrochemical cell that derives electrical energy from spontaneous redox reactions taking place within the cell.
It was invented by italian physicist alessandro volta who published his experiments in 1799.
A battery is a device consisting of one or more electrochemical cells with external connections for powering electrical devices such as flashlights mobile phones and electric cars. Volta was the inventor of the voltaic pile the first electrical battery. Voltaic cells are typically used as a source of electrical power. It consists of two separate half cells. When a battery is supplying electric power its positive terminal is the cathode. Unlike most nuclear power sources which use nuclear radiation to generate heat which then is used to generate electricity betavoltaic devices use a non thermal conversion process converting the electron hole pairs produced by the ionization trail of beta particles traversing.

We consider alessandro volta s voltaic pile as the first wet battery cell. A battery is a set of voltaic cells that are connected in parallel. A betavoltaic device is a type of nuclear battery which generates electric current from beta particles emitted from a radioactive source using semiconductor junctions. A galvanic cell or voltaic cell named after luigi galvani or alessandro volta respectively is an electrochemical cell that derives electrical energy from spontaneous redox reactions taking place within the cell. This system could produce a measurable current.
 Source: pinterest.com
Source: pinterest.com
However it wasn t until the work of luigi galvani in the late 1700s that battery technology was modernized. Thus the history of battery began. It generally consists of two different metals immersed in electrolytes or of individual half cells with different metals and their ions in solution connected by a salt bridge or separated by a porous membrane. A betavoltaic device is a type of nuclear battery which generates electric current from beta particles emitted from a radioactive source using semiconductor junctions. It is named for its inventor alessandro volta who built the first example in 1800 and was based upon previous work by luigi galvani.
 Source: alamy.com
Source: alamy.com
A betavoltaic device is a type of nuclear battery which generates electric current from beta particles emitted from a radioactive source using semiconductor junctions. A battery is a set of voltaic cells that are connected in parallel. The voltaic pile then enabled a rapid series of other discoveries including the electrical decomposition of water into oxygen and hydrogen by william nicholson and anthony carlisle and the discovery or isolation of the chemical elements sodium potassium calcium boron barium strontium. We consider alessandro volta s voltaic pile as the first wet battery cell. It consists of two separate half cells.
 Source: researchgate.net
Source: researchgate.net
A galvanic cell or voltaic cell named after luigi galvani or alessandro volta respectively is an electrochemical cell that derives electrical energy from spontaneous redox reactions taking place within the cell. A simple and reliable source of electric current that did not need. By their nature they produce direct current. A voltaic cell also known as a galvanic cell is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A betavoltaic device is a type of nuclear battery which generates electric current from beta particles emitted from a radioactive source using semiconductor junctions.
 Source: sparkmuseum.org
Source: sparkmuseum.org
It originated as a schematic drawing of the earliest type of battery a voltaic pile. It generally consists of two different metals immersed in electrolytes or of individual half cells with different metals and their ions in solution connected by a salt bridge or separated by a porous membrane. Volta was the inventor of the voltaic pile the first electrical battery. After that in 1800 he developed the first voltaic cell battery constructed of alternating copper and zinc discs with pieces of cardboard soaked in brine between them. A battery is a device consisting of one or more electrochemical cells with external connections for powering electrical devices such as flashlights mobile phones and electric cars.
 Source: nationalmaglab.org
Source: nationalmaglab.org
Unlike most nuclear power sources which use nuclear radiation to generate heat which then is used to generate electricity betavoltaic devices use a non thermal conversion process converting the electron hole pairs produced by the ionization trail of beta particles traversing. Unlike most nuclear power sources which use nuclear radiation to generate heat which then is used to generate electricity betavoltaic devices use a non thermal conversion process converting the electron hole pairs produced by the ionization trail of beta particles traversing. A battery is a set of voltaic cells that are connected in parallel. We consider alessandro volta s voltaic pile as the first wet battery cell. A battery is a device consisting of one or more electrochemical cells with external connections for powering electrical devices such as flashlights mobile phones and electric cars.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A common source used is the hydrogen isotope tritium. It was invented by italian physicist alessandro volta who published his experiments in 1799. A voltaic pile is the first type of true electric battery capable of a sustained output of electric current. Unlike most nuclear power sources which use nuclear radiation to generate heat which then is used to generate electricity betavoltaic devices use a non thermal conversion process converting the electron hole pairs produced by the ionization trail of beta particles traversing. A battery is a device consisting of one or more electrochemical cells with external connections for powering electrical devices such as flashlights mobile phones and electric cars.
 Source: libraries.mit.edu
Source: libraries.mit.edu
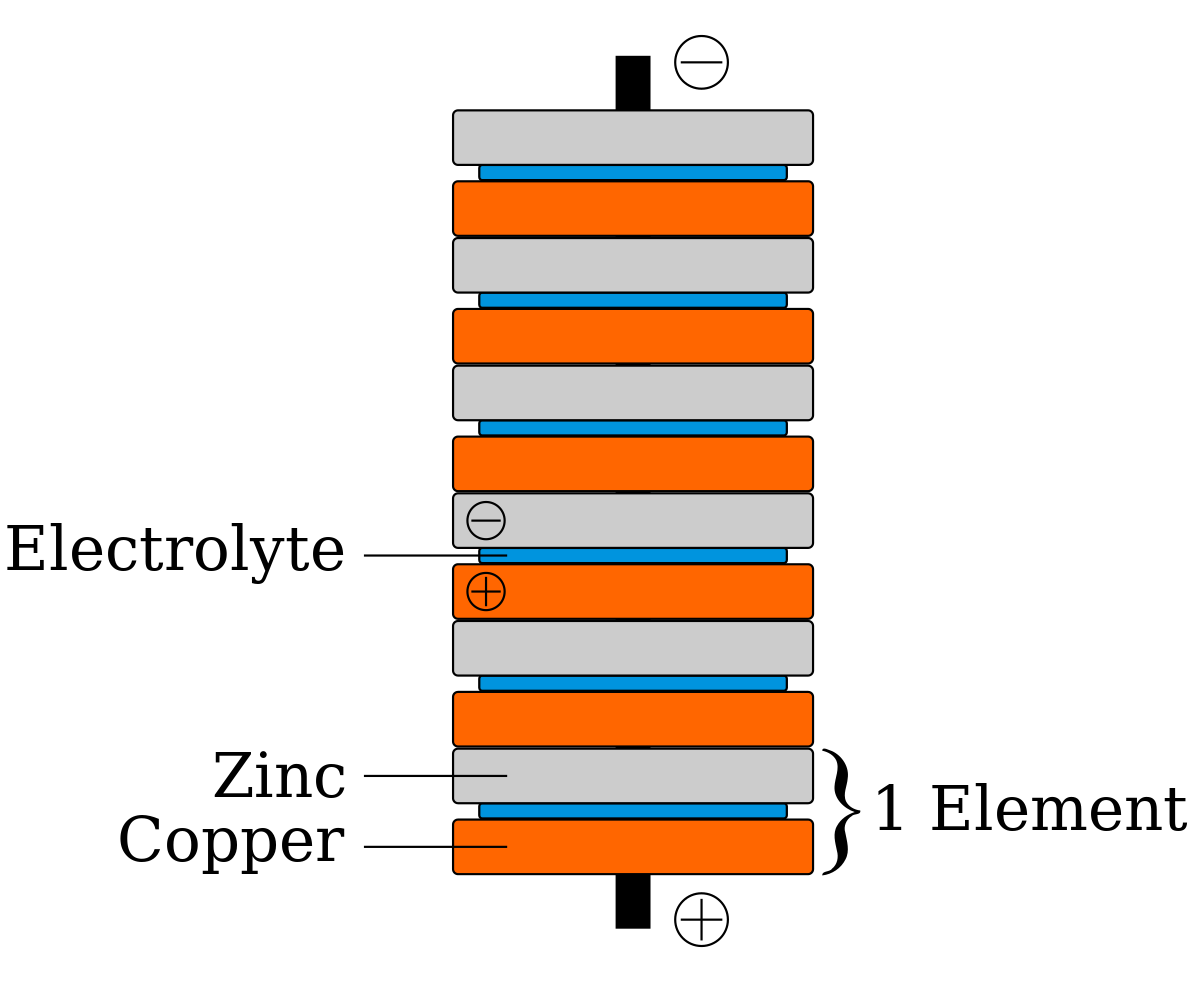
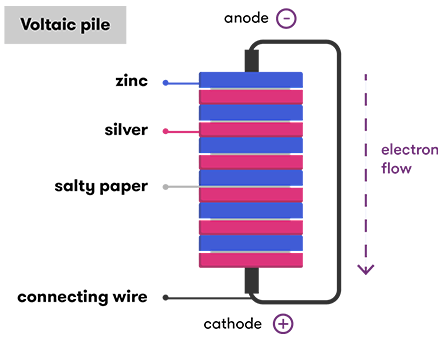
When a battery is supplying electric power its positive terminal is the cathode. A galvanic cell or voltaic cell named after luigi galvani or alessandro volta respectively is an electrochemical cell that derives electrical energy from spontaneous redox reactions taking place within the cell. Known as the voltaic pile or the voltaic column volta s battery consisted of alternating disks of zinc and silver or copper and pewter separated by paper or cloth soaked either in salt water or sodium hydroxide. After that in 1800 he developed the first voltaic cell battery constructed of alternating copper and zinc discs with pieces of cardboard soaked in brine between them. It consists of two separate half cells.
 Source: nationalmaglab.org
Source: nationalmaglab.org
A voltaic pile is the first type of true electric battery capable of a sustained output of electric current. Thus the history of battery began. The use of the voltaic cell appears to date back to ancient times with the discovery of the baghdad battery in 1936 a series of galvanic cells placed in large jars from sometime in the first few centuries ad. A battery is a set of voltaic cells that are connected in parallel. Unlike most nuclear power sources which use nuclear radiation to generate heat which then is used to generate electricity betavoltaic devices use a non thermal conversion process converting the electron hole pairs produced by the ionization trail of beta particles traversing.
 Source: science.org.au
Source: science.org.au
A battery is a set of voltaic cells that are connected in parallel. By their nature they produce direct current. After that in 1800 he developed the first voltaic cell battery constructed of alternating copper and zinc discs with pieces of cardboard soaked in brine between them. A voltaic pile is the first type of true electric battery capable of a sustained output of electric current. Voltaic cells are typically used as a source of electrical power.
 Source: libraries.mit.edu
Source: libraries.mit.edu
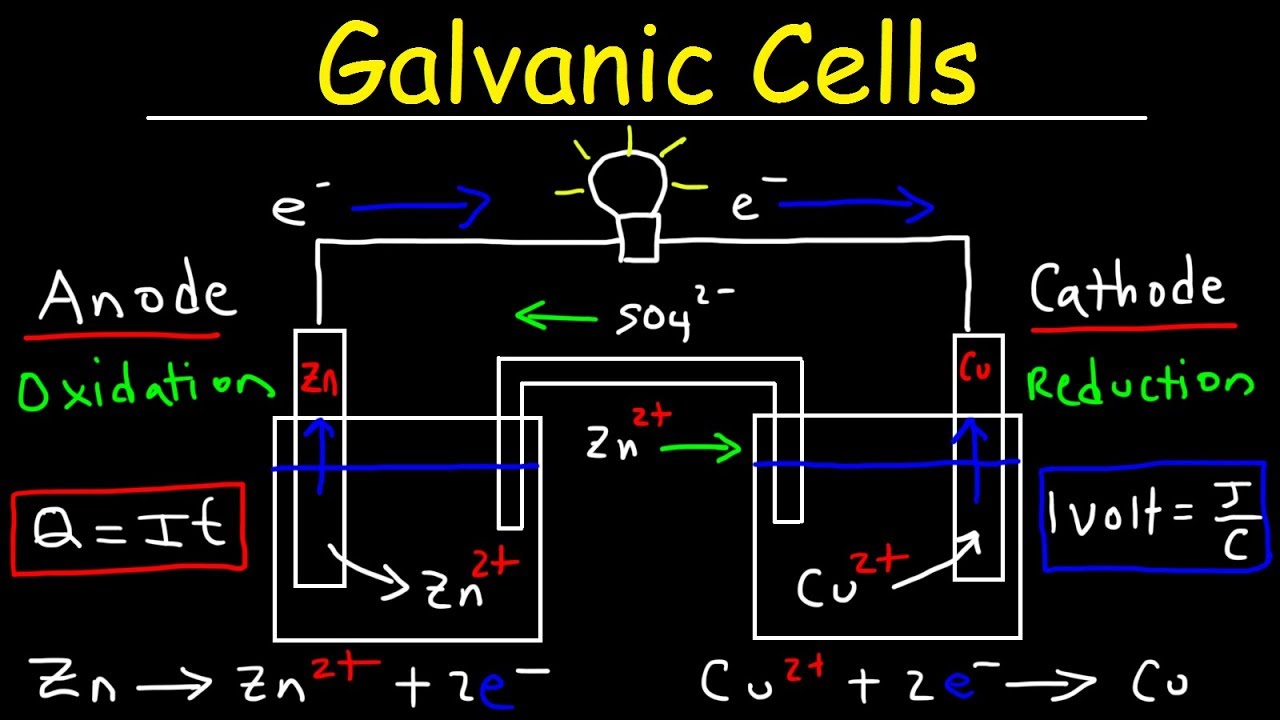
By their nature they produce direct current. A battery is a device consisting of one or more electrochemical cells with external connections for powering electrical devices such as flashlights mobile phones and electric cars. Known as the voltaic pile or the voltaic column volta s battery consisted of alternating disks of zinc and silver or copper and pewter separated by paper or cloth soaked either in salt water or sodium hydroxide. It consists of two separate half cells. The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit.
 Source: pinterest.com
Source: pinterest.com
The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit. A battery is a set of voltaic cells that are connected in parallel. Known as the voltaic pile or the voltaic column volta s battery consisted of alternating disks of zinc and silver or copper and pewter separated by paper or cloth soaked either in salt water or sodium hydroxide. Voltaic cells are typically used as a source of electrical power. A half cell is composed of an electrode a strip of metal m within a solution containing m n ions in which m is any arbitrary metal.
 Source: dkfindout.com
Source: dkfindout.com
For instance a lead acid battery has cells with the anodes composed of lead and cathodes composed of lead dioxide. A battery is a set of voltaic cells that are connected in parallel. Known as the voltaic pile or the voltaic column volta s battery consisted of alternating disks of zinc and silver or copper and pewter separated by paper or cloth soaked either in salt water or sodium hydroxide. The voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit. It generally consists of two different metals immersed in electrolytes or of individual half cells with different metals and their ions in solution connected by a salt bridge or separated by a porous membrane.
 Source: electronics.howstuffworks.com
Source: electronics.howstuffworks.com
After that in 1800 he developed the first voltaic cell battery constructed of alternating copper and zinc discs with pieces of cardboard soaked in brine between them. A simple and reliable source of electric current that did not need. By their nature they produce direct current. A common source used is the hydrogen isotope tritium. We consider alessandro volta s voltaic pile as the first wet battery cell.
 Source: collection.sciencemuseumgroup.org.uk
Source: collection.sciencemuseumgroup.org.uk
Unlike most nuclear power sources which use nuclear radiation to generate heat which then is used to generate electricity betavoltaic devices use a non thermal conversion process converting the electron hole pairs produced by the ionization trail of beta particles traversing. We consider alessandro volta s voltaic pile as the first wet battery cell. It is named for its inventor alessandro volta who built the first example in 1800 and was based upon previous work by luigi galvani. The use of the voltaic cell appears to date back to ancient times with the discovery of the baghdad battery in 1936 a series of galvanic cells placed in large jars from sometime in the first few centuries ad. It was invented by italian physicist alessandro volta who published his experiments in 1799.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what is a voltaic battery by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.