What gets plated during electroplating
What Gets Plated During Electroplating. Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. The sbe process is accomplished in a chamber with ultrasonic action and continuous solution being pumped into and out of the chamber to facilitate part movement and supply fresh plating electrolyte during the plating process. Jewelry and silverware can be silver or gold plated while zinc is often used to coat iron to protect against rust. The process involves passing an electric current through a solution of electrolytes that allows for the transfer of metal ions from the donor metal to the recipient metal.
 Electroplating Wikipedia From en.wikipedia.org
Electroplating Wikipedia From en.wikipedia.org
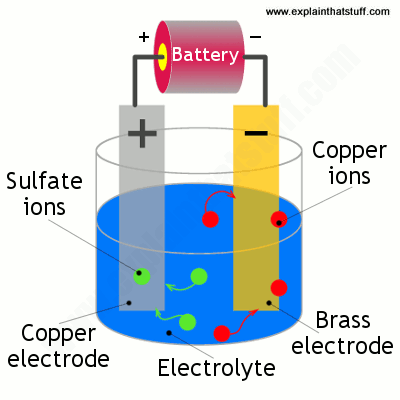
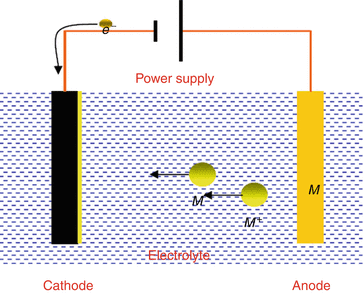
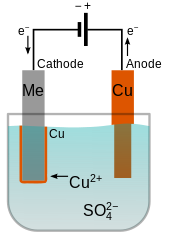
A simple example of the electroplating process is the electroplating of copper in which the metal to be plated copper is used as the anode and the electrolyte solution contains the ion of the metal to be plated cu 2 in this example. The sbe process is accomplished in a chamber with ultrasonic action and continuous solution being pumped into and out of the chamber to facilitate part movement and supply fresh plating electrolyte during the plating process. Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal. Electroplating or electroless plating may be used as a way to render a metal part radioactive by using an aqueous solution prepared from nickel phosphorus concentrates which contain radioactive hypophosphite 32 p ions. Setting up a typical electrolytic cell for electroplating.
Alloys such as brass and bronze can be plated too by arranging for the electrolyte to contain salts of all the metals that need to be present in the alloy.
The object to be plated coated with a different metal is made the cathode negative electrode by connecting it to the negative terminal of the power supply. Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. Tin plated steel is chromium plated to prevent dulling of the surface due to oxidation of tin. A kinds of metals can be electroplated such as gold silver tin zinc copper cadmium chromium platinum and lead what is silver plated zinc. Alloys such as brass and bronze can be plated too by arranging for the electrolyte to contain salts of all the metals that need to be present in the alloy. Jewelry and silverware can be silver or gold plated while zinc is often used to coat iron to protect against rust.
 Source: explainthatstuff.com
Source: explainthatstuff.com
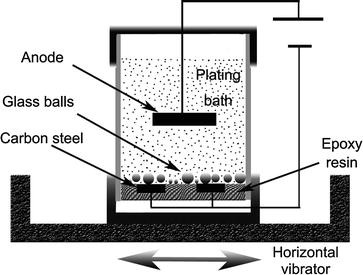
The sbe process is accomplished in a chamber with ultrasonic action and continuous solution being pumped into and out of the chamber to facilitate part movement and supply fresh plating electrolyte during the plating process. Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal. As most of us learned in elementary. What gets plated in electroplating. The object to be plated coated with a different metal is made the cathode negative electrode by connecting it to the negative terminal of the power supply.
 Source: britannica.com
Source: britannica.com
Copper goes into solution at the anode as it is plated at the cathode. The sbe process is accomplished in a chamber with ultrasonic action and continuous solution being pumped into and out of the chamber to facilitate part movement and supply fresh plating electrolyte during the plating process. The object to be plated coated with a different metal is made the cathode negative electrode by connecting it to the negative terminal of the power supply. There are many objects in our everyday lives that have been plated with other metals. As most of us learned in elementary.
 Source: byjus.com
Source: byjus.com
Solutions containing metal ions are placed in a tank and the piece of metal to be plated is connected to the electrical supply to become the cathode. Setting up a typical electrolytic cell for electroplating. Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal. Electroplating is a chemical change. Electroplating is essentially a chemical reaction which helps to make various items we see and use every day.
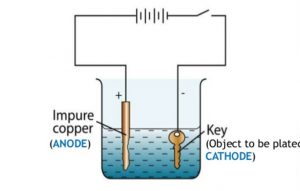 Source: classnotes.org.in
Source: classnotes.org.in
Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. The object to be plated coated with a different metal is made the cathode negative electrode by connecting it to the negative terminal of the power supply. Electroplating is also used for making duplicates of printing plates in a process called electrotyping and for electroforming an alternative to casting objects from molten metals. Gold plated jewelry nickel and copper plated coins etc. Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal.
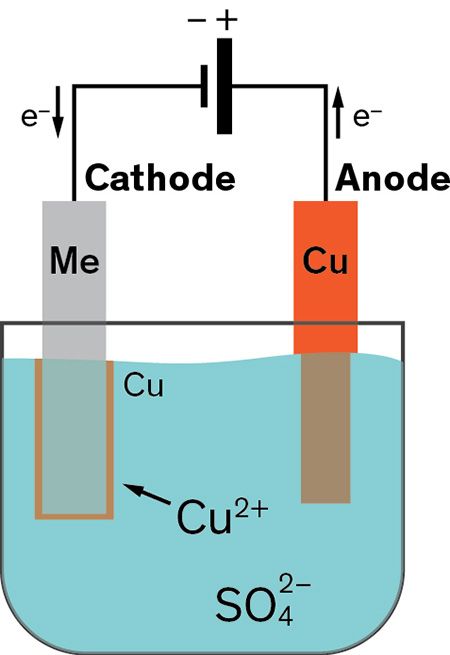
The process involves passing an electric current through a solution of electrolytes that allows for the transfer of metal ions from the donor metal to the recipient metal. What gets plated in electroplating. Alloys such as brass and bronze can be plated too by arranging for the electrolyte to contain salts of all the metals that need to be present in the alloy. Electroplating or electroless plating may be used as a way to render a metal part radioactive by using an aqueous solution prepared from nickel phosphorus concentrates which contain radioactive hypophosphite 32 p ions. There are many objects in our everyday lives that have been plated with other metals.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Electroplating is essentially a chemical reaction which helps to make various items we see and use every day. Electroplating is a chemical change. Alloys such as brass and bronze can be plated too by arranging for the electrolyte to contain salts of all the metals that need to be present in the alloy. As most of us learned in elementary. Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal.
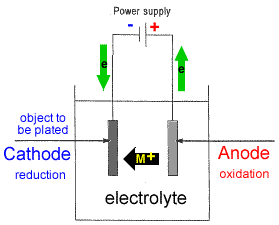 Source: ausetute.com.au
Source: ausetute.com.au
Copper goes into solution at the anode as it is plated at the cathode. Tin plated steel is chromium plated to prevent dulling of the surface due to oxidation of tin. A simple example of the electroplating process is the electroplating of copper in which the metal to be plated copper is used as the anode and the electrolyte solution contains the ion of the metal to be plated cu 2 in this example. Jewelry and silverware can be silver or gold plated while zinc is often used to coat iron to protect against rust. The sbe ensures very uniform plating coverage in high and low current density areas of the part as well as in counter bores.

Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal. Tin plated steel is chromium plated to prevent dulling of the surface due to oxidation of tin. There are many objects in our everyday lives that have been plated with other metals. Jewelry and silverware can be silver or gold plated while zinc is often used to coat iron to protect against rust. What gets plated in electroplating.
 Source: quora.com
Source: quora.com
The process involves passing an electric current through a solution of electrolytes that allows for the transfer of metal ions from the donor metal to the recipient metal. There are many objects in our everyday lives that have been plated with other metals. The object to be plated coated with a different metal is made the cathode negative electrode by connecting it to the negative terminal of the power supply. Jewelry and silverware can be silver or gold plated while zinc is often used to coat iron to protect against rust. Copper goes into solution at the anode as it is plated at the cathode.
 Source: thoughtco.com
Source: thoughtco.com
Electroplating or electroless plating may be used as a way to render a metal part radioactive by using an aqueous solution prepared from nickel phosphorus concentrates which contain radioactive hypophosphite 32 p ions. A simple example of the electroplating process is the electroplating of copper in which the metal to be plated copper is used as the anode and the electrolyte solution contains the ion of the metal to be plated cu 2 in this example. There are many objects in our everyday lives that have been plated with other metals. As most of us learned in elementary. The process involves passing an electric current through a solution of electrolytes that allows for the transfer of metal ions from the donor metal to the recipient metal.
 Source: link.springer.com
Source: link.springer.com
There are many objects in our everyday lives that have been plated with other metals. However the primary bath component is something we all use every day. Gold plated jewelry nickel and copper plated coins etc. Solutions containing metal ions are placed in a tank and the piece of metal to be plated is connected to the electrical supply to become the cathode. What you need to know the electroplating process involves the use of an aqueous electrolyte solution known as the plating bath that consists of dissolved metal salts ions and various chemicals.
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
Electroplating or electroless plating may be used as a way to render a metal part radioactive by using an aqueous solution prepared from nickel phosphorus concentrates which contain radioactive hypophosphite 32 p ions. Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal. Copper goes into solution at the anode as it is plated at the cathode. Alloys such as brass and bronze can be plated too by arranging for the electrolyte to contain salts of all the metals that need to be present in the alloy. What gets plated in electroplating.
 Source: docbrown.info
Source: docbrown.info
Setting up a typical electrolytic cell for electroplating. As most of us learned in elementary. What you need to know the electroplating process involves the use of an aqueous electrolyte solution known as the plating bath that consists of dissolved metal salts ions and various chemicals. Hydrogen embrittlement electroplating. Copper goes into solution at the anode as it is plated at the cathode.
 Source: link.springer.com
Source: link.springer.com
Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal. Jewelry and silverware can be silver or gold plated while zinc is often used to coat iron to protect against rust. As most of us learned in elementary. Solutions containing metal ions are placed in a tank and the piece of metal to be plated is connected to the electrical supply to become the cathode. The sbe ensures very uniform plating coverage in high and low current density areas of the part as well as in counter bores.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Gold plated jewelry nickel and copper plated coins etc. As most of us learned in elementary. Electroplating uses an electrolytic cell to deposit a coating of one metal onto another metal. Solutions containing metal ions are placed in a tank and the piece of metal to be plated is connected to the electrical supply to become the cathode. Copper goes into solution at the anode as it is plated at the cathode.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what gets plated during electroplating by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





