What are the products of the electrolysis of water
What Are The Products Of The Electrolysis Of Water. Products of electrolysis during electrolysis the reactions occurring at the electrodes are oxidation and reduction reactions. The products of electrolysis can be predicted for a given electrolyte. In water salt actually splits into na and cl ions which are very good at carrying current or the flow of electric charges. The electrolysis of water produces hydrogen and oxygen gases.
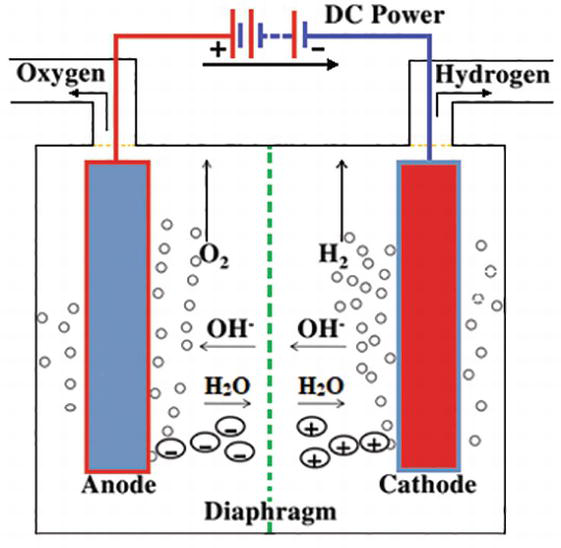 Hydrogen Generation By Water Electrolysis Intechopen From intechopen.com
Hydrogen Generation By Water Electrolysis Intechopen From intechopen.com
Products of electrolysis during electrolysis the reactions occurring at the electrodes are oxidation and reduction reactions. In water salt actually splits into na and cl ions which are very good at carrying current or the flow of electric charges. Electrolysis of water is the decomposition of water h 2 o into oxygen o 2 and hydrogen gas h 2 due to an electric current being passed through the water. The products of electrolysis can be predicted for a given electrolyte. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h 2 so 4 has been added. The electrolysis of water produces hydrogen and oxygen gases.
The key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit.
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. The electrolysis of water produces hydrogen and oxygen gases. Table salt or sodium chloride nacl is also a good additive to form electrolytes. In chloralkali process sodium chloride nacl is the feedstock in the form of a saturated aqueous solution whereas chlorine is the main product and hydrogen is the by product. Electrolysis of water is its decomposition to give hydrogen and oxygen gases due to the passage of an electric current. The products of electrolysis depend on the nature of material being electrolysed and the types of electrodes being used if the electrode is inert such as gold or platinum it does not take part in the chemical reaction and acts only as a source or sink for electrons.
 Source: docbrown.info
Source: docbrown.info
The products of electrolysis depend on the nature of material being electrolysed and the types of electrodes being used if the electrode is inert such as gold or platinum it does not take part in the chemical reaction and acts only as a source or sink for electrons. The products of electrolysis can be predicted for a given electrolyte. This electrolytic process is used in some industrial applications when hydrogen is needed. The products of electrolysis depend on the nature of material being electrolysed and the types of electrodes being used if the electrode is inert such as gold or platinum it does not take part in the chemical reaction and acts only as a source or sink for electrons. In chloralkali process sodium chloride nacl is the feedstock in the form of a saturated aqueous solution whereas chlorine is the main product and hydrogen is the by product.
 Source: intechopen.com
Source: intechopen.com
Electrolysis of water is its decomposition to give hydrogen and oxygen gases due to the passage of an electric current. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. Products of electrolysis during electrolysis the reactions occurring at the electrodes are oxidation and reduction reactions. The products of electrolysis can be predicted for a given electrolyte. The main components of an electrolytic cell are an electrolyte dc current and two electrodes.
 Source: docbrown.info
Source: docbrown.info
The key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. In water salt actually splits into na and cl ions which are very good at carrying current or the flow of electric charges. In chloralkali process sodium chloride nacl is the feedstock in the form of a saturated aqueous solution whereas chlorine is the main product and hydrogen is the by product. The products of electrolysis can be predicted for a given electrolyte.
 Source: sciencedirect.com
Source: sciencedirect.com
In the water baking soda solution the gases that are produced are hydrogen h 2 oxygen o 2 and carbon dioxide co 2. The main components of an electrolytic cell are an electrolyte dc current and two electrodes. The electrolysis of water produces hydrogen and oxygen gases. Electrolysis involves using electricity to break down electrolytes to form elements. This electrolytic process is used in some industrial applications when hydrogen is needed.

Electrolysis of water 2h2o electrical energy o2 2h2. Electrolysis of water 2h2o electrical energy o2 2h2. This electrolytic process is used in some industrial applications when hydrogen is needed. The electrolysis of water produces hydrogen and oxygen gases. The main components of an electrolytic cell are an electrolyte dc current and two electrodes.
 Source: meritnation.com
Source: meritnation.com
Table salt or sodium chloride nacl is also a good additive to form electrolytes. In the water baking soda solution the gases that are produced are hydrogen h 2 oxygen o 2 and carbon dioxide co 2. In water salt actually splits into na and cl ions which are very good at carrying current or the flow of electric charges. Electrolysis involves using electricity to break down electrolytes to form elements. This electrolytic process is used in some industrial applications when hydrogen is needed.
 Source: docbrown.info
Source: docbrown.info
The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h 2 so 4 has been added. Products of electrolysis during electrolysis the reactions occurring at the electrodes are oxidation and reduction reactions. This electrolytic process is used in some industrial applications when hydrogen is needed. Electrolysis involves using electricity to break down electrolytes to form elements. In the water baking soda solution the gases that are produced are hydrogen h 2 oxygen o 2 and carbon dioxide co 2.
 Source: phys.org
Source: phys.org
In water salt actually splits into na and cl ions which are very good at carrying current or the flow of electric charges. In the water baking soda solution the gases that are produced are hydrogen h 2 oxygen o 2 and carbon dioxide co 2. Electrolysis of water is its decomposition to give hydrogen and oxygen gases due to the passage of an electric current. The key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. In water salt actually splits into na and cl ions which are very good at carrying current or the flow of electric charges.
 Source: docbrown.info
Source: docbrown.info
The products of electrolysis depend on the nature of material being electrolysed and the types of electrodes being used if the electrode is inert such as gold or platinum it does not take part in the chemical reaction and acts only as a source or sink for electrons. Products of electrolysis during electrolysis the reactions occurring at the electrodes are oxidation and reduction reactions. Electrolysis involves using electricity to break down electrolytes to form elements. Electrolysis of water is the decomposition of water h 2 o into oxygen o 2 and hydrogen gas h 2 due to an electric current being passed through the water. The main components of an electrolytic cell are an electrolyte dc current and two electrodes.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Electrolysis involves using electricity to break down electrolytes to form elements. In water salt actually splits into na and cl ions which are very good at carrying current or the flow of electric charges. The key process of electrolysis is the interchange of atoms and ions by the removal or addition of electrons to the external circuit. In water electrolysis pure water is the feedstock to produce hydrogen as the main product and oxygen as the by product. This electrolytic process is used in some industrial applications when hydrogen is needed.
 Source: sciencedirect.com
Source: sciencedirect.com
Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h 2 so 4 has been added. Electrolysis of water is the decomposition of water h 2 o into oxygen o 2 and hydrogen gas h 2 due to an electric current being passed through the water. Table salt or sodium chloride nacl is also a good additive to form electrolytes. The products of electrolysis can be predicted for a given electrolyte.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Products of electrolysis during electrolysis the reactions occurring at the electrodes are oxidation and reduction reactions. The main components of an electrolytic cell are an electrolyte dc current and two electrodes. This electrolytic process is used in some industrial applications when hydrogen is needed. Electrolysis of water 2h2o electrical energy o2 2h2. In chloralkali process sodium chloride nacl is the feedstock in the form of a saturated aqueous solution whereas chlorine is the main product and hydrogen is the by product.
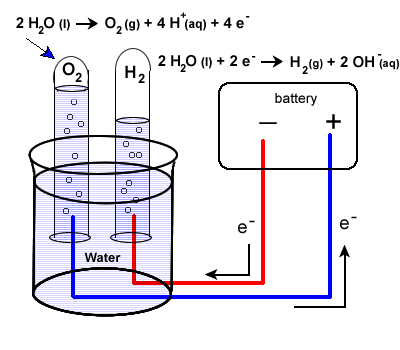 Source: chem.libretexts.org
Source: chem.libretexts.org
In chloralkali process sodium chloride nacl is the feedstock in the form of a saturated aqueous solution whereas chlorine is the main product and hydrogen is the by product. Electrolysis of water is its decomposition to give hydrogen and oxygen gases due to the passage of an electric current. In water electrolysis pure water is the feedstock to produce hydrogen as the main product and oxygen as the by product. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as h 2 so 4 has been added. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas.
 Source: youtube.com
Source: youtube.com
The main components of an electrolytic cell are an electrolyte dc current and two electrodes. In the water baking soda solution the gases that are produced are hydrogen h 2 oxygen o 2 and carbon dioxide co 2. The main components of an electrolytic cell are an electrolyte dc current and two electrodes. Electrolysis of water 2h2o electrical energy o2 2h2. This electrolytic process is used in some industrial applications when hydrogen is needed.
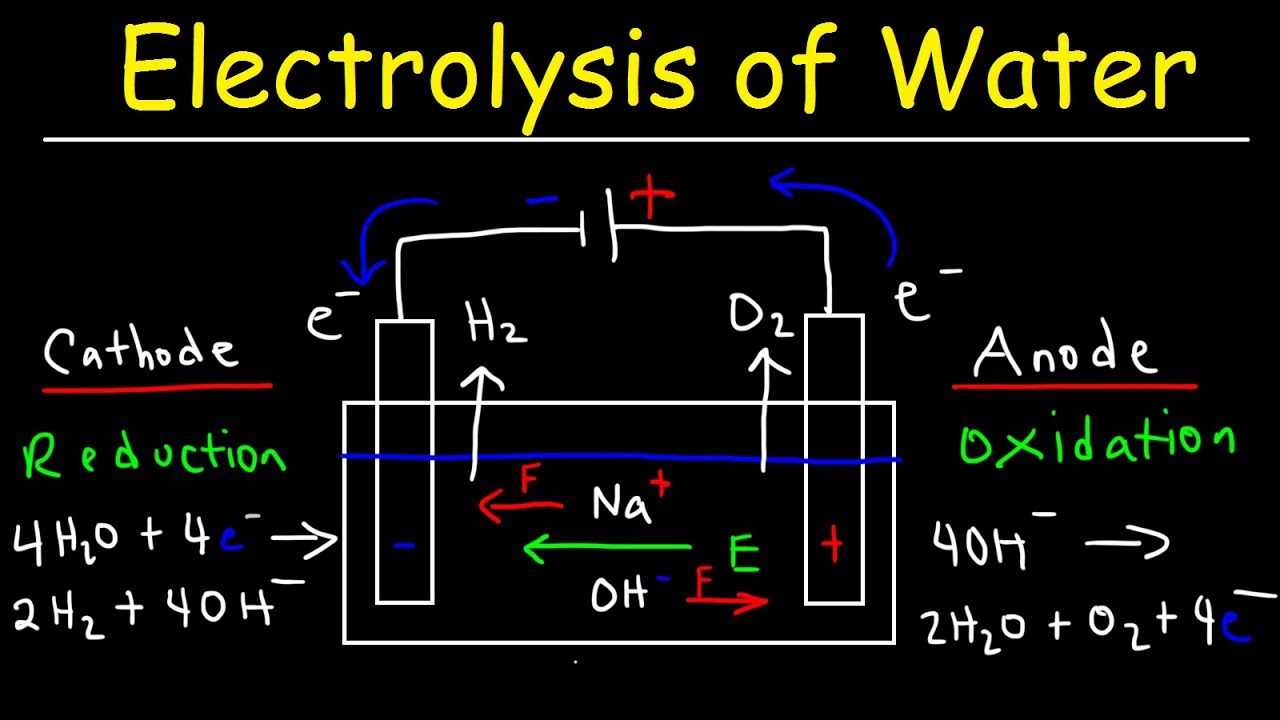 Source: youtube.com
Source: youtube.com
The electrolysis of water produces hydrogen and oxygen gases. Table salt or sodium chloride nacl is also a good additive to form electrolytes. The electrolysis of water produces hydrogen and oxygen gases. This electrolytic process is used in some industrial applications when hydrogen is needed. Electrolysis of water 2h2o electrical energy o2 2h2.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what are the products of the electrolysis of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





