Water molecule and polarity
Water Molecule And Polarity. Polarity of a water molecule. Oxygen has a very high electronegativity meaning it has a very high affinity for electrons. The geometry in turn is due to oxygen s two lone pairs. The ocs molecule has a structure similar to co 2 but a sulfur atom has replaced one of the oxygen atoms.
 Why Most People Don T Understand Anything About Water From newwatermodel.blogspot.com
Why Most People Don T Understand Anything About Water From newwatermodel.blogspot.com
This is an example of polar covalent chemical bonding. A water molecule consists of one oxygen atom and two hydrogen atoms. The ocs molecule has a structure similar to co 2 but a sulfur atom has replaced one of the oxygen atoms. Vsepr theory predicts a linear molecule. The c o bond is considerably polar. To determine if this molecule is polar we draw the molecular structure.
The polarity of a molecule majorly depends on its constituent atoms and their arrangement around the central atom.
Water is a polar molecule since it is formed using a strongly electronegative oxygen atom that pulls a pair of the hydrogen atoms and possesses a slightly negative charge on it. This is an example of polar covalent chemical bonding. This causes on end of the molecule to be negative while the other is positive. B in contrast water is polar because the oh bond moments do not cancel out. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. The end of the molecule with the two hydrogen atoms is positively charged.
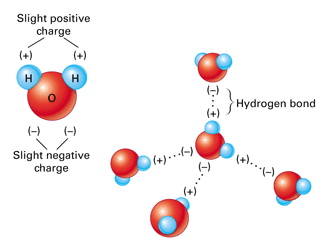 Source: bodell.mtchs.org
Source: bodell.mtchs.org
When the two hydrogen atoms bond with the oxygen they attach to the top of the molecule rather like mickey mouse ears. A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. The intermolecular forces between the slightly positively charged end of one molecule to the negative end of another or the same molecule. This causes on end of the molecule to be negative while the other is positive. The end of the molecule with the two hydrogen atoms is positively charged.
 Source: sciencenotes.org
Source: sciencenotes.org
This causes on end of the molecule to be negative while the other is positive. The ocs molecule has a structure similar to co 2 but a sulfur atom has replaced one of the oxygen atoms. Polarity of a water molecule. A water molecule consists of one oxygen atom and two hydrogen atoms. Vsepr theory predicts a linear molecule.
 Source: newwatermodel.blogspot.com
Source: newwatermodel.blogspot.com
B in contrast water is polar because the oh bond moments do not cancel out. This molecular structure gives the water molecule polarity or a lopsided electrical charge that attracts other atoms. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. The intermolecular forces between the slightly positively charged end of one molecule to the negative end of another or the same molecule. B in contrast water is polar because the oh bond moments do not cancel out.
 Source: khanacademy.org
Source: khanacademy.org
The c o bond is considerably polar. When solutes are added to water they may be affected by the charge distribution. Polarity of a water molecule. The oxygen in water molecules pulls the electrons from the hydrogen atoms closer to it creating two poles in the molecule where the hydrogen end is partially positive and the oxygen end is partially negative. The ocs molecule has a structure similar to co 2 but a sulfur atom has replaced one of the oxygen atoms.
 Source: biology.arizona.edu
Source: biology.arizona.edu
The two hydrogen atoms and one oxygen atom within water molecules h 2 o form polar covalent bonds. The end of the molecule with the two hydrogen atoms is positively charged. The intermolecular forces between the slightly positively charged end of one molecule to the negative end of another or the same molecule. A water molecule consists of one oxygen atom and two hydrogen atoms. This molecular structure gives the water molecule polarity or a lopsided electrical charge that attracts other atoms.
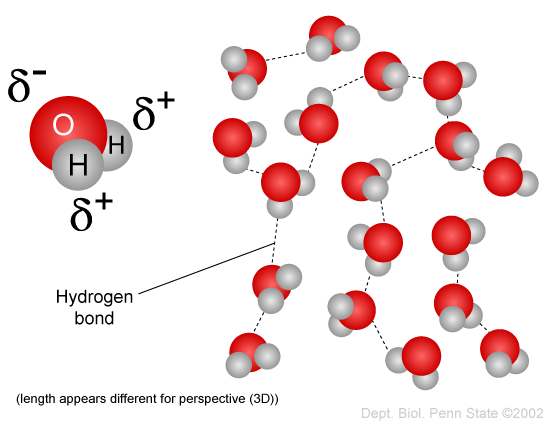 Source: sphweb.bumc.bu.edu
Source: sphweb.bumc.bu.edu
A water molecule is formed by a combination of two hydrogen atoms and one oxygen atom. B in contrast water is polar because the oh bond moments do not cancel out. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen. When the two hydrogen atoms bond with the oxygen they attach to the top of the molecule rather like mickey mouse ears. The intermolecular forces between the slightly positively charged end of one molecule to the negative end of another or the same molecule.
 Source: usgs.gov
Source: usgs.gov
Water h 2 o is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. The end of the molecule with the two hydrogen atoms is positively charged. Water h 2 o is polar because of the bent shape of the molecule. When the two hydrogen atoms bond with the oxygen they attach to the top of the molecule rather like mickey mouse ears.
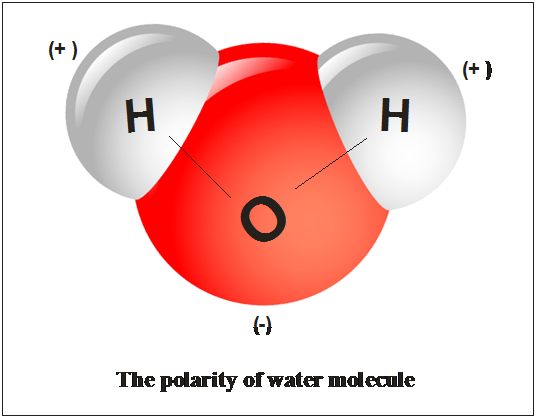 Source: chegg.com
Source: chegg.com
The oxygen in water molecules pulls the electrons from the hydrogen atoms closer to it creating two poles in the molecule where the hydrogen end is partially positive and the oxygen end is partially negative. Water h 2 o is a polar molecule and a polar solvent. The end of the molecule with the two hydrogen atoms is positively charged. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen.
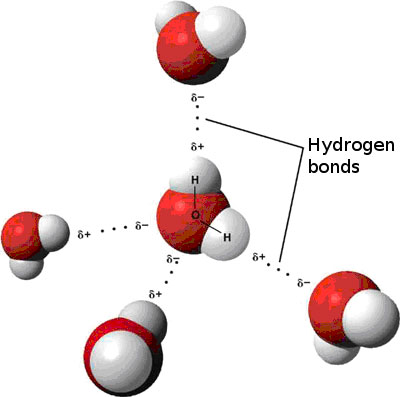 Source: sciencepartners.info
Source: sciencepartners.info
The intermolecular forces between the slightly positively charged end of one molecule to the negative end of another or the same molecule. One of water s important properties is that it is composed of polar molecules. The ocs molecule has a structure similar to co 2 but a sulfur atom has replaced one of the oxygen atoms. Water h 2 o is a polar molecule and a polar solvent. Vsepr theory predicts a linear molecule.
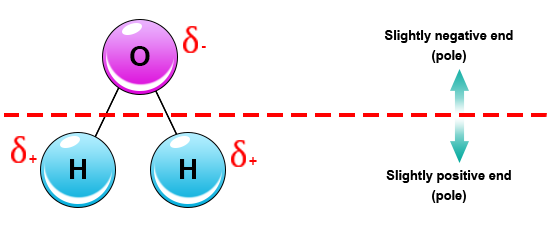 Source: socratic.org
Source: socratic.org
Water is a polar molecule and polarity occurs when the electrons in molecules are not spread evenly. When solutes are added to water they may be affected by the charge distribution. While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen. The geometry in turn is due to oxygen s two lone pairs. Vsepr theory predicts a linear molecule.
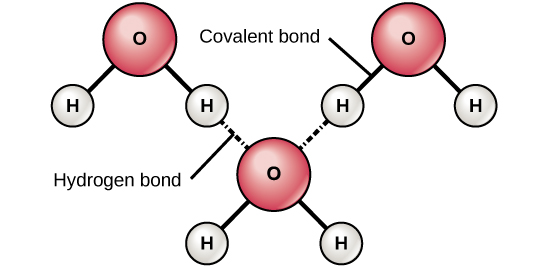 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
While there is no net charge to a water molecule the polarity of water creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen. The oxygen in water molecules pulls the electrons from the hydrogen atoms closer to it creating two poles in the molecule where the hydrogen end is partially positive and the oxygen end is partially negative. One of water s important properties is that it is composed of polar molecules. When solutes are added to water they may be affected by the charge distribution. A water molecule consists of one oxygen atom and two hydrogen atoms.
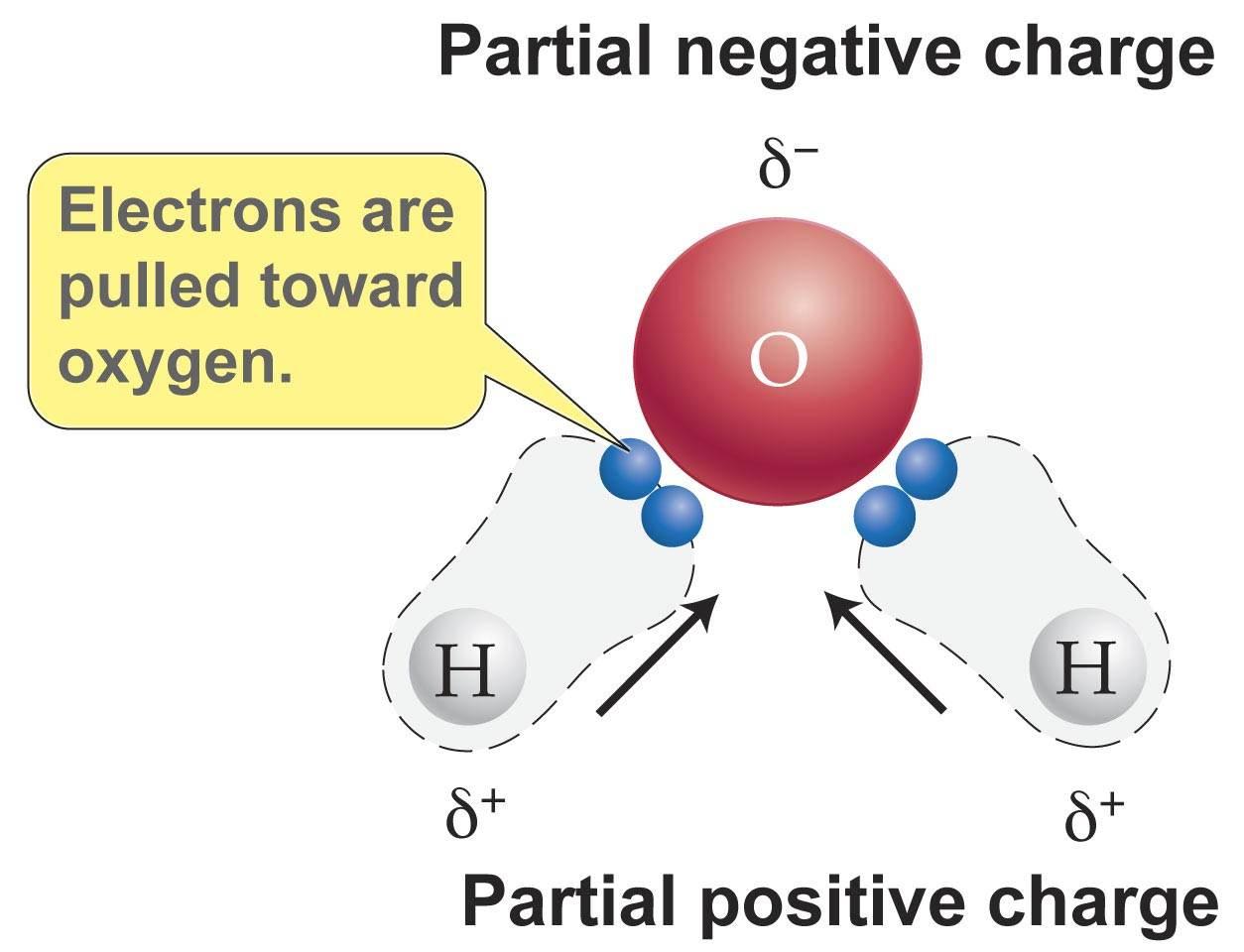 Source: socratic.org
Source: socratic.org
When solutes are added to water they may be affected by the charge distribution. The geometry in turn is due to oxygen s two lone pairs. This causes on end of the molecule to be negative while the other is positive. Water is a polar molecule since it is formed using a strongly electronegative oxygen atom that pulls a pair of the hydrogen atoms and possesses a slightly negative charge on it. B in contrast water is polar because the oh bond moments do not cancel out.
 Source: sites.google.com
Source: sites.google.com
Water is a polar molecule since it is formed using a strongly electronegative oxygen atom that pulls a pair of the hydrogen atoms and possesses a slightly negative charge on it. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. The hydrogen atom bonds to each oxygen atom with a pair of shared electrons. Water is a polar molecule since it is formed using a strongly electronegative oxygen atom that pulls a pair of the hydrogen atoms and possesses a slightly negative charge on it. To determine if this molecule is polar we draw the molecular structure.
 Source: usgs.gov
Source: usgs.gov
The c o bond is considerably polar. B in contrast water is polar because the oh bond moments do not cancel out. One of water s important properties is that it is composed of polar molecules. This molecular structure gives the water molecule polarity or a lopsided electrical charge that attracts other atoms. Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms.
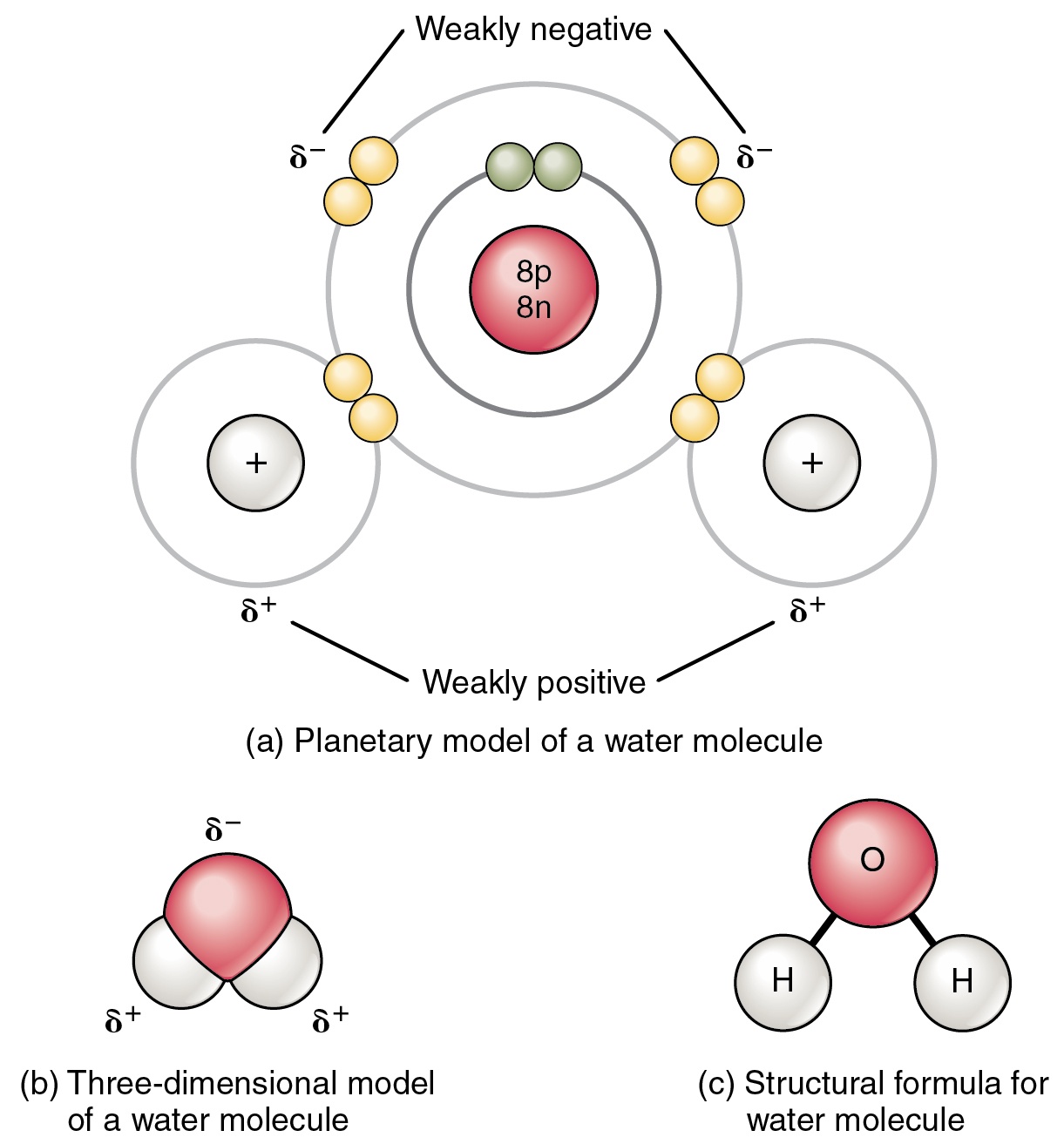 Source: expii.com
Source: expii.com
Water is a polar molecule because of its bent geometry and the electronegativity difference between the hydrogen and oxygen atoms. One of water s important properties is that it is composed of polar molecules. The end of the molecule with the two hydrogen atoms is positively charged. Water is a polar molecule since it is formed using a strongly electronegative oxygen atom that pulls a pair of the hydrogen atoms and possesses a slightly negative charge on it. The intermolecular forces between the slightly positively charged end of one molecule to the negative end of another or the same molecule.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title water molecule and polarity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






