Water is a polar molecule what does this mean
Water Is A Polar Molecule What Does This Mean. Water h 2 o is a polar molecule and a polar solvent. Updated may 06 2019. As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. Since the water molecule comprising two atoms of hydrogen and one of oxygen is formed by covalent bonds the electrons are shared.
 Polar And Non Polar Molecules By Ron Kurtus Understanding Chemistry School For Champions From school-for-champions.com
Polar And Non Polar Molecules By Ron Kurtus Understanding Chemistry School For Champions From school-for-champions.com
As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. In diagrams the lowercase letter delta δ shows the charge distribution in a polar molecule. Water h 2 o is a polar molecule and a polar solvent. What does this mean. The positive charge comes from the atomic nucleus while the electrons supply the negative charge. Water is a polar molecule and also acts as a polar solvent.
The positive charge comes from the atomic nucleus while the electrons supply the negative charge.
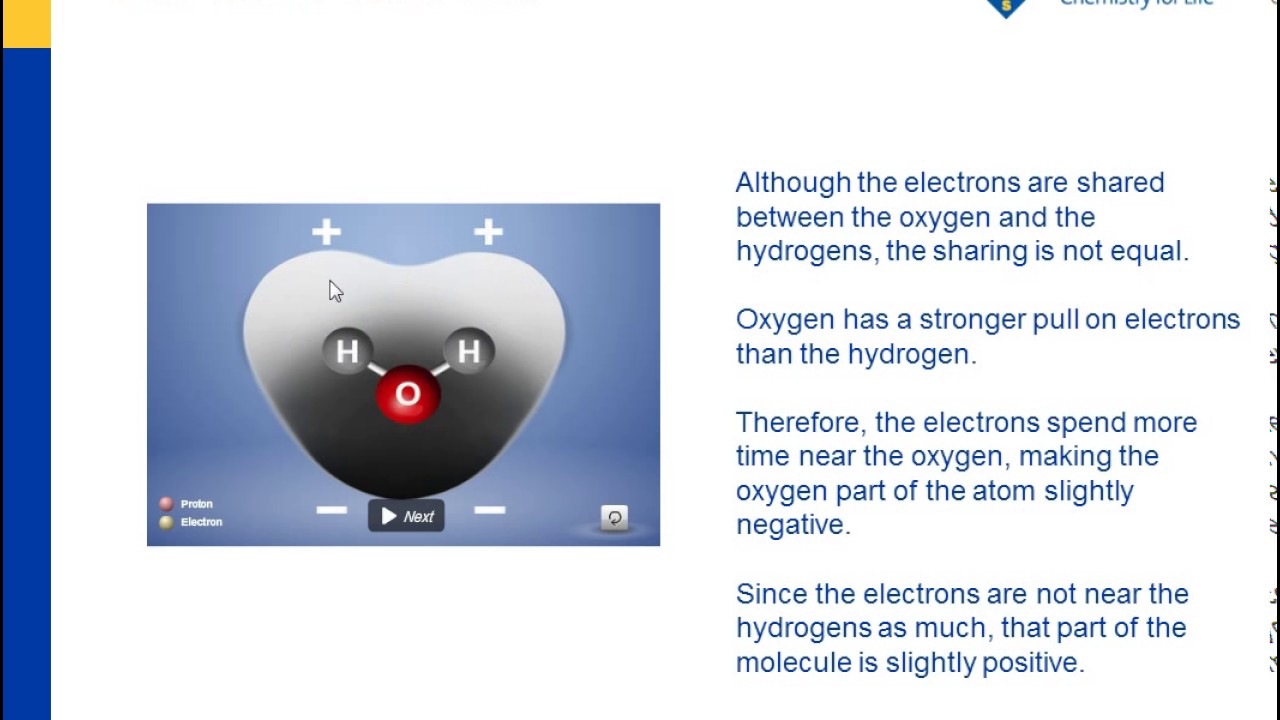
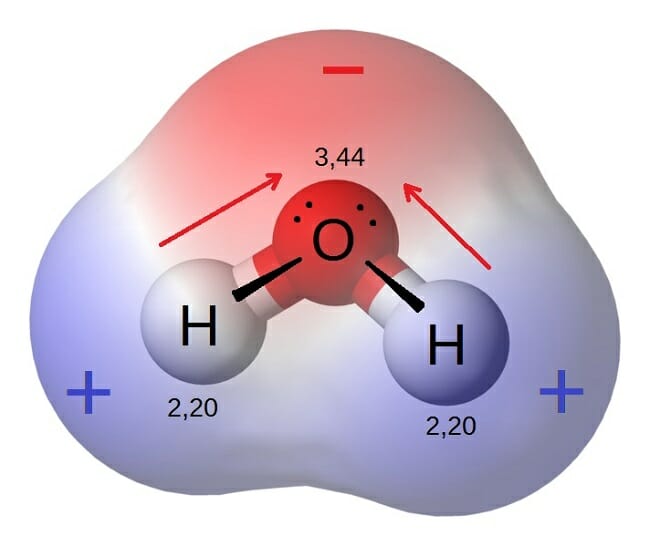
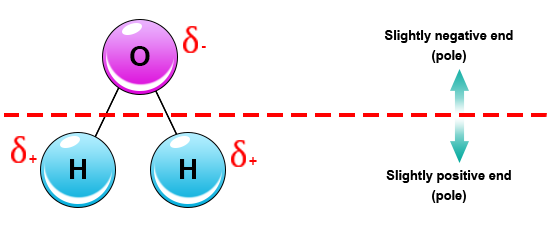
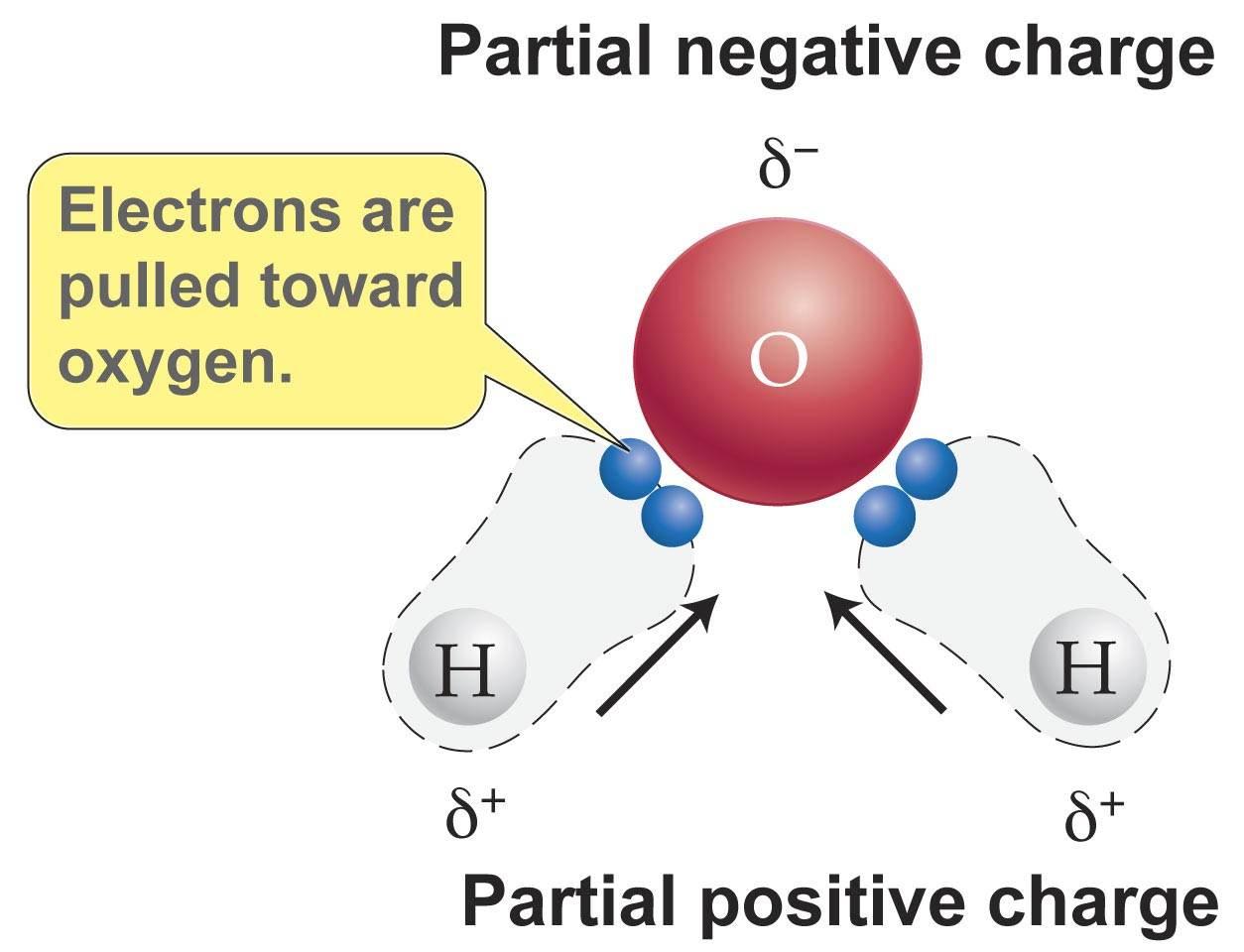
As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. This sharing causes the electrons to stay closer to the oxygen atom giving it a slight negative charge while the hydrogen atoms as a consequence have a slight positive charge. This forces most of the electrons to the side of the molecule where oxygen is present creating a highly negative area. In diagrams the lowercase letter delta δ shows the charge distribution in a polar molecule. As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. When a molecule is polar it means its positive and negative electrical charges are unevenly distributed so part of the molecule is partially positive while part is partially negative.
 Source: study.com
Source: study.com
When a molecule is polar it means its positive and negative electrical charges are unevenly distributed so part of the molecule is partially positive while part is partially negative. When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. The most important polar molecule on earth is water. What does this mean. In diagrams the lowercase letter delta δ shows the charge distribution in a polar molecule.
 Source: sciencenotes.org
Source: sciencenotes.org
Water is a polar molecule and also acts as a polar solvent. This sharing causes the electrons to stay closer to the oxygen atom giving it a slight negative charge while the hydrogen atoms as a consequence have a slight positive charge. In diagrams the lowercase letter delta δ shows the charge distribution in a polar molecule. Updated may 06 2019. Water h 2 o is a polar molecule and a polar solvent.
 Source: sites.google.com
Source: sites.google.com
What does this mean. Since the water molecule comprising two atoms of hydrogen and one of oxygen is formed by covalent bonds the electrons are shared. This sharing causes the electrons to stay closer to the oxygen atom giving it a slight negative charge while the hydrogen atoms as a consequence have a slight positive charge. Water is a polar molecule and also acts as a polar solvent. In diagrams the lowercase letter delta δ shows the charge distribution in a polar molecule.
 Source: sciencenotes.org
Source: sciencenotes.org
Water is a polar molecule and also acts as a polar solvent. As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. Water is a polar molecule and also acts as a polar solvent. This forces most of the electrons to the side of the molecule where oxygen is present creating a highly negative area. Since the water molecule comprising two atoms of hydrogen and one of oxygen is formed by covalent bonds the electrons are shared.
 Source: slideplayer.com
Source: slideplayer.com
The positive charge comes from the atomic nucleus while the electrons supply the negative charge. Water h 2 o is a polar molecule and a polar solvent. The most important polar molecule on earth is water. When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. Since the water molecule comprising two atoms of hydrogen and one of oxygen is formed by covalent bonds the electrons are shared.
 Source: biology.arizona.edu
Source: biology.arizona.edu
Water h 2 o is a polar molecule and a polar solvent. When a molecule is polar it means its positive and negative electrical charges are unevenly distributed so part of the molecule is partially positive while part is partially negative. As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. Updated may 06 2019. The most important polar molecule on earth is water.
 Source: quora.com
Source: quora.com
When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. The most important polar molecule on earth is water. This forces most of the electrons to the side of the molecule where oxygen is present creating a highly negative area. Water h 2 o is a polar molecule and a polar solvent. In diagrams the lowercase letter delta δ shows the charge distribution in a polar molecule.
 Source: slideplayer.com
Source: slideplayer.com
Since the water molecule comprising two atoms of hydrogen and one of oxygen is formed by covalent bonds the electrons are shared. This sharing causes the electrons to stay closer to the oxygen atom giving it a slight negative charge while the hydrogen atoms as a consequence have a slight positive charge. The positive charge comes from the atomic nucleus while the electrons supply the negative charge. This forces most of the electrons to the side of the molecule where oxygen is present creating a highly negative area. The most important polar molecule on earth is water.
 Source: youtube.com
Source: youtube.com
Updated may 06 2019. What does this mean. The most important polar molecule on earth is water. In diagrams the lowercase letter delta δ shows the charge distribution in a polar molecule. The positive charge comes from the atomic nucleus while the electrons supply the negative charge.
 Source: khanacademy.org
Source: khanacademy.org
Water is a polar molecule and also acts as a polar solvent. As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. Water is a polar molecule and also acts as a polar solvent. Water h 2 o is a polar molecule and a polar solvent. The most important polar molecule on earth is water.
 Source: biologydictionary.net
Source: biologydictionary.net
Water is a polar molecule and also acts as a polar solvent. This sharing causes the electrons to stay closer to the oxygen atom giving it a slight negative charge while the hydrogen atoms as a consequence have a slight positive charge. Updated may 06 2019. The positive charge comes from the atomic nucleus while the electrons supply the negative charge. Water is a polar molecule and also acts as a polar solvent.
 Source: socratic.org
Source: socratic.org
Updated may 06 2019. As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. In diagrams the lowercase letter delta δ shows the charge distribution in a polar molecule. This sharing causes the electrons to stay closer to the oxygen atom giving it a slight negative charge while the hydrogen atoms as a consequence have a slight positive charge. This forces most of the electrons to the side of the molecule where oxygen is present creating a highly negative area.
 Source: littlesciencequestions.wordpress.com
Source: littlesciencequestions.wordpress.com
As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. Water h 2 o is a polar molecule and a polar solvent. As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. When a molecule is polar it means its positive and negative electrical charges are unevenly distributed so part of the molecule is partially positive while part is partially negative.
 Source: school-for-champions.com
Source: school-for-champions.com
Since the water molecule comprising two atoms of hydrogen and one of oxygen is formed by covalent bonds the electrons are shared. This forces most of the electrons to the side of the molecule where oxygen is present creating a highly negative area. In diagrams the lowercase letter delta δ shows the charge distribution in a polar molecule. Water h 2 o is a polar molecule and a polar solvent. What does this mean.
 Source: socratic.org
Source: socratic.org
Water is a polar molecule and also acts as a polar solvent. This sharing causes the electrons to stay closer to the oxygen atom giving it a slight negative charge while the hydrogen atoms as a consequence have a slight positive charge. When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. As seen in the image below water is a polar molecule due to the strong electronegativity of the oxygen atom. The positive charge comes from the atomic nucleus while the electrons supply the negative charge.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title water is a polar molecule what does this mean by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






