Universal solvent property of water
Universal Solvent Property Of Water. Water is the universal solvent. Water is the substance that we refer to as the universal solvent. Amphoteric nature of water. Water is described as the universal solvent.
 Good Solvent Water Properties Examples Expii From expii.com
Good Solvent Water Properties Examples Expii From expii.com
The main reason is its ability to donate and accept protons. Water molecules are made of two hydrogens and one oxygen. Water is capable of dissolving a variety of different substances which is why it is such a good solvent. The bonds which hold the hydrogen and oxygen together are called covalent bonds they are very strong. Water is the substance that we refer to as the universal solvent. A solvent is simply a liquid that other substances can dissolve in and the reason that water has gained the label of universal solvent is because no other solvent can dissolve as many substances as it can.
H2oone molecule of water is comprised of 2 atoms of hydrogen and one atom of oxygen bonded together.
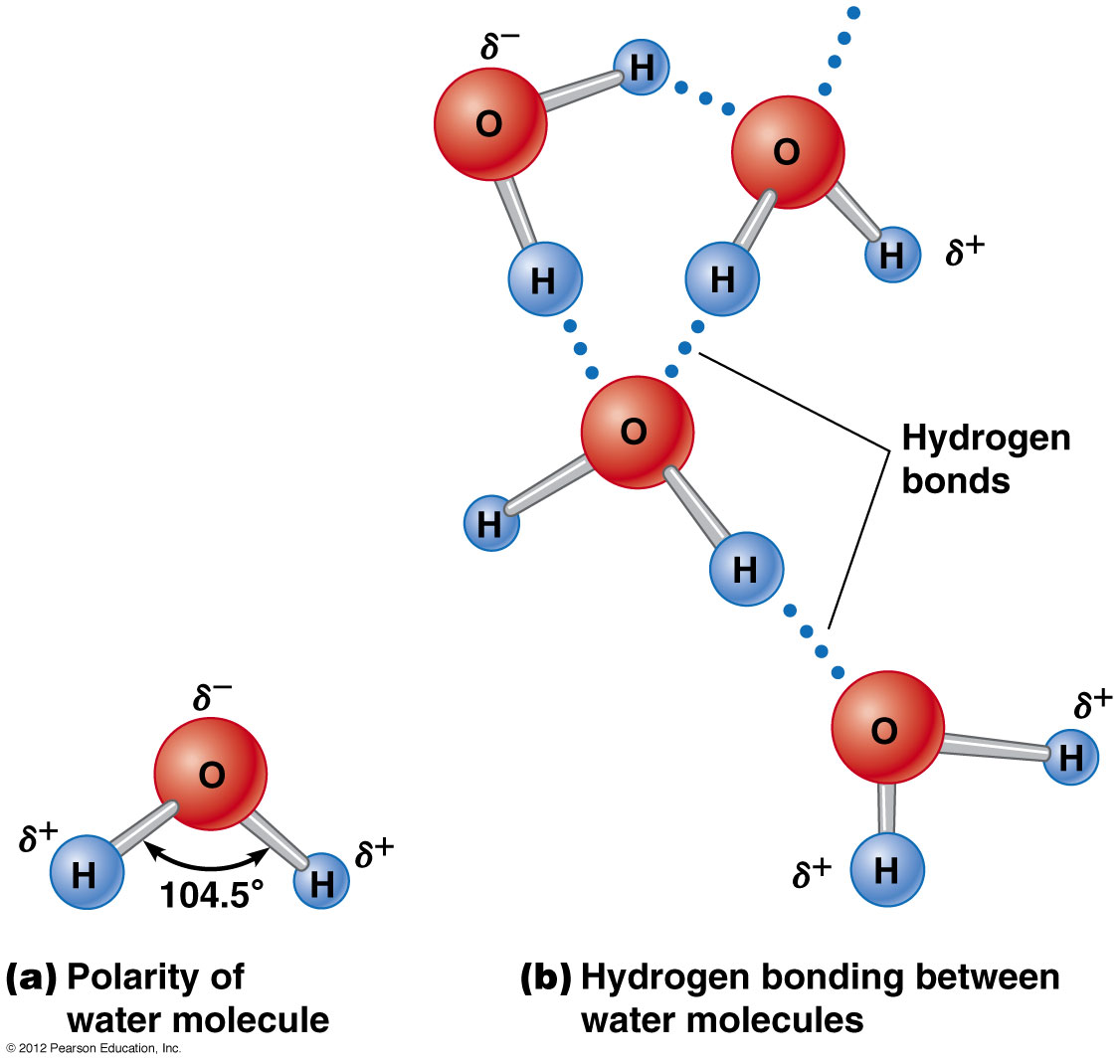
This unique property of water is due to hydrogen bonding. These atoms are of different sizes and charges which creates the asymmetry in the molecular structure and leads to strong bonds between water and other polar molecules including water itself. This unique property of water is due to hydrogen bonding. H2oone molecule of water is comprised of 2 atoms of hydrogen and one atom of oxygen bonded together. The hydrogen side of each water h 2 o molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge. Water is the substance that we refer to as the universal solvent.
 Source: usgs.gov
Source: usgs.gov
This has to do with the polarity of each water molecule. The bonds which hold the hydrogen and oxygen together are called covalent bonds they are very strong. This unique property of water is due to hydrogen bonding. Water molecules are made of two hydrogens and one oxygen. This has to do with the polarity of each water molecule.
 Source: e-education.psu.edu
Source: e-education.psu.edu
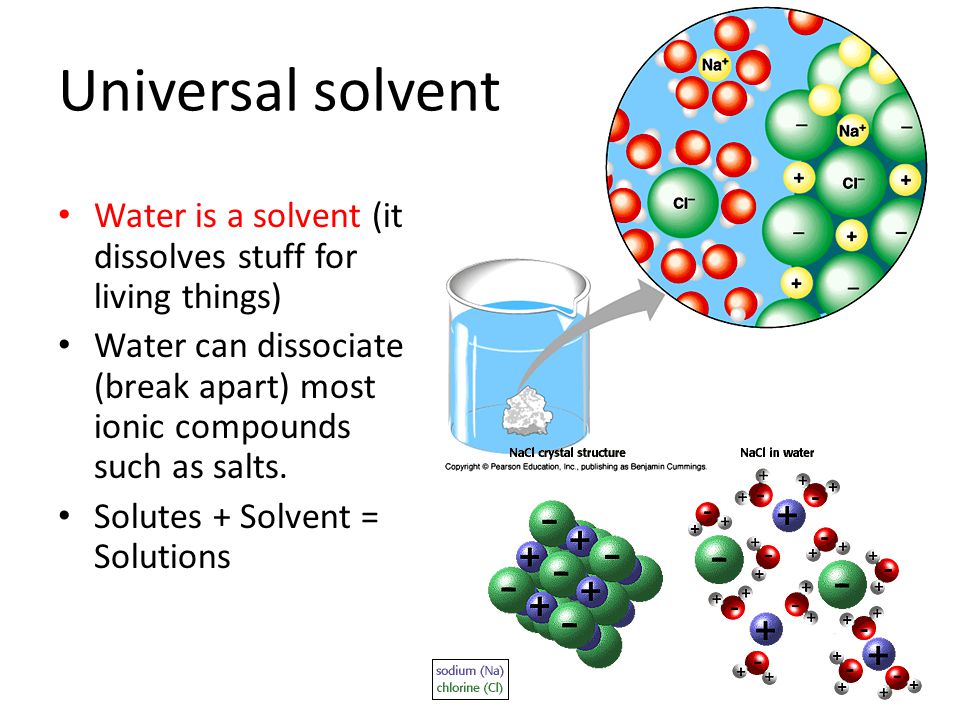
Water s solvent properties water which not only dissolves many compounds but also dissolves more substances than any other liquid is considered the universal solvent. This is important to every living thing on earth. Water molecules are made of two hydrogens and one oxygen. Amphoteric means the ability of the substance to act as an acid or base. This is because of the chemical composition and physical attributes.
 Source: slideplayer.com
Source: slideplayer.com
These atoms are of different sizes and charges which creates the asymmetry in the molecular structure and leads to strong bonds between water and other polar molecules including water itself. This unique property of water is due to hydrogen bonding. And water is called the universal solvent because it dissolves more substances than any other liquid. Water is the universal solvent. The molecules of water are constantly moving concerning each other and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds 2 10 13 seconds.
 Source: socratic.org
Source: socratic.org
Water is capable of dissolving a variety of different substances which is why it is such a good solvent. The amphoteric nature is one of the most important properties of water. This helps water dissociate ionic compounds into their positive and negative ions. The universal solvent 1 water the universal solvent 2 water is a chemical. And water is called the universal solvent because it dissolves more substances than any other liquid.
 Source: biologydictionary.net
Source: biologydictionary.net
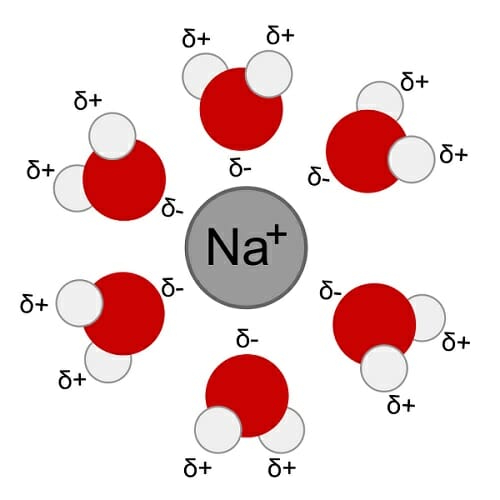
The hydrogen side of each water h 2 o molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge. Water molecules are made of two hydrogens and one oxygen. The hydrogen side of each water h 2 o molecule carries a slight positive electric charge while the oxygen side carries a slight negative electric charge. This is important to every living thing on earth. Water s solvent properties water which not only dissolves many compounds but also dissolves more substances than any other liquid is considered the universal solvent.
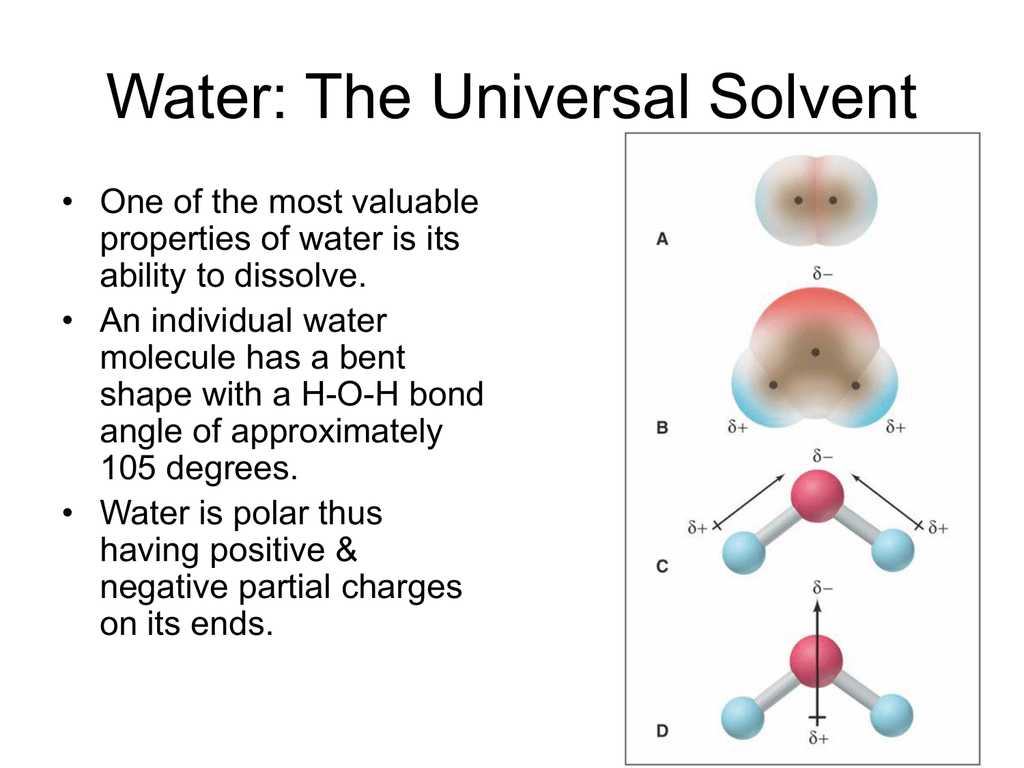 Source: studylib.net
Source: studylib.net
The bonds which hold the hydrogen and oxygen together are called covalent bonds they are very strong. The molecules of water are constantly moving concerning each other and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds 2 10 13 seconds. Water is called the universal solvent because it is capable of dissolving more substances than any other liquid. This is because of the chemical composition and physical attributes. Amphoteric means the ability of the substance to act as an acid or base.
 Source: m.youtube.com
Source: m.youtube.com
This helps water dissociate ionic compounds into their positive and negative ions. Why is water the universal solvent. These atoms are of different sizes and charges which creates the asymmetry in the molecular structure and leads to strong bonds between water and other polar molecules including water itself. Water s solvent properties water which not only dissolves many compounds but also dissolves more substances than any other liquid is considered the universal solvent. Water is the universal solvent.
 Source: usgs.gov
Source: usgs.gov
Water is capable of dissolving a variety of different substances which is why it is such a good solvent. Water is the substance that we refer to as the universal solvent. This unique property of water is due to hydrogen bonding. Water molecules are made of two hydrogens and one oxygen. Solvent properties of water thanks to its ability to dissolve a wide range of solutes water is sometimes called the universal solvent however this name isn t entirely accurate since there are some substances such as oils that don t dissolve well in water.
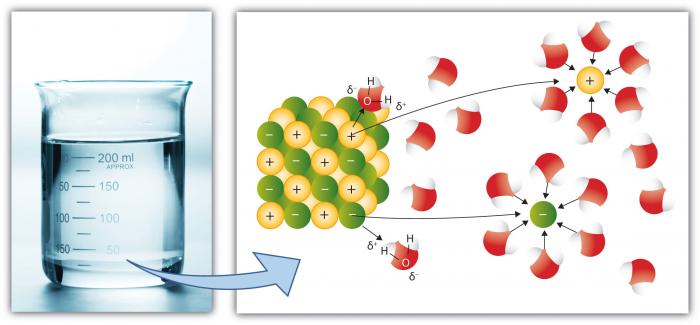 Source: e-education.psu.edu
Source: e-education.psu.edu
Water is the universal solvent. A polar molecule with partially positive and negative charges it readily dissolves ions and polar molecules. This unique property of water is due to hydrogen bonding. Solvent properties of water thanks to its ability to dissolve a wide range of solutes water is sometimes called the universal solvent however this name isn t entirely accurate since there are some substances such as oils that don t dissolve well in water. Water in the natural state is neither acidic nor basic.
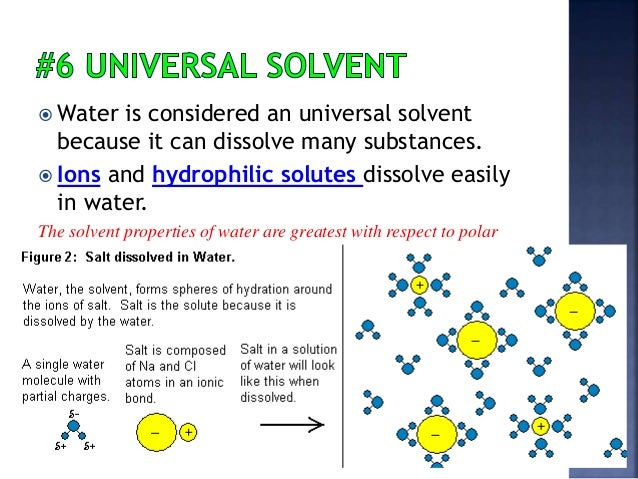 Source: slideshare.net
Source: slideshare.net
A solvent is simply a liquid that other substances can dissolve in and the reason that water has gained the label of universal solvent is because no other solvent can dissolve as many substances as it can. Water is called the universal solvent because more substances dissolve in water than in any other chemical. The universal solvent 1 water the universal solvent 2 water is a chemical. The molecules of water are constantly moving concerning each other and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds 2 10 13 seconds. Amphoteric nature of water.
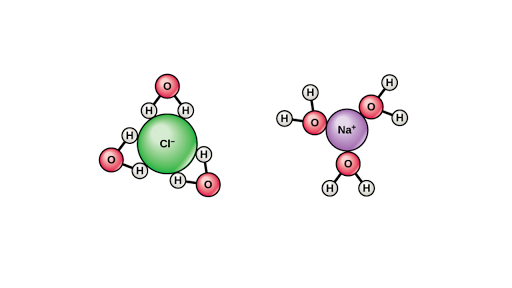
Water is called the universal solvent because more substances dissolve in water than in any other chemical. Water is called the universal solvent because it is capable of dissolving more substances than any other liquid. The amphoteric nature is one of the most important properties of water. 3 water as a solvent. Water is described as the universal solvent.
 Source: studylib.net
Source: studylib.net
The universal solvent 1 water the universal solvent 2 water is a chemical. The molecules of water are constantly moving concerning each other and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds 2 10 13 seconds. This is because of the chemical composition and physical attributes. Water is called the universal solvent because more substances dissolve in water than in any other chemical. Amphoteric nature of water.
 Source: expii.com
Source: expii.com
This has to do with the polarity of each water molecule. This is important to every living thing on earth. Water molecules are made of two hydrogens and one oxygen. A solvent is simply a liquid that other substances can dissolve in and the reason that water has gained the label of universal solvent is because no other solvent can dissolve as many substances as it can. The universal solvent 1 water the universal solvent 2 water is a chemical.
 Source: thoughtco.com
Source: thoughtco.com
Amphoteric nature of water. This is important to every living thing on earth. The bonds which hold the hydrogen and oxygen together are called covalent bonds they are very strong. Water in the natural state is neither acidic nor basic. However rainwater is slightly acidic with a ph between 5 2 and 5 8.
 Source: ib.bioninja.com.au
Source: ib.bioninja.com.au
And water is called the universal solvent because it dissolves more substances than any other liquid. It means that wherever water goes either through the air the ground or through our bodies it takes along valuable chemicals minerals and nutrients. This is because of the chemical composition and physical attributes. This unique property of water is due to hydrogen bonding. Why is water the universal solvent.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title universal solvent property of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.