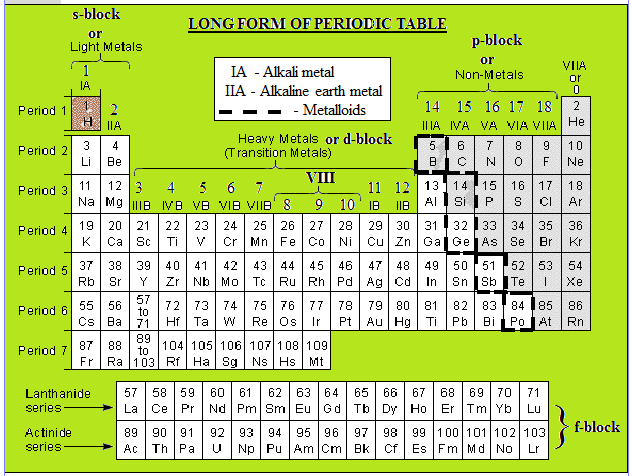
Transition metal characteristics
Transition Metal Characteristics. Multiple oxidation states since there is a low energy gap between them. They are generally good conductors of heat and electricity and tend to crystallize in bcc body centred cubic ccp cubic close packed or hcp hexagonally close packed structures. Transition metals demonstrate a wide range of chemical behaviors. The transition metals exhibit typical metallic properties such as malleability ductility high tensile strength and metallic lustre.
What Is The Definition Of Inner Transition Elements Quora From quora.com
They are generally good conductors of heat and electricity and tend to crystallize in bcc body centred cubic ccp cubic close packed or hcp hexagonally close packed structures. They are good conductors of heat and electricity they can be hammered or bent into shape easily. Quick summary of the transition metal properties. The transition metals have the following physical properties. Transition metals demonstrate a wide range of chemical behaviors. For example the lanthanides all form stable 3 aqueous cations.
The transition metals have the following physical properties.
Quick summary of the transition metal properties. Transition metal any of various chemical elements that have valence electrons i e electrons that can participate in the formation of chemical bonds in two shells instead of only one. The transition metals have the following physical properties. Quick summary of the transition metal properties. Multiple oxidation states since there is a low energy gap between them. As can be seen from their reduction potentials table p1 some transition metals are strong reducing agents whereas others have very low reactivity.
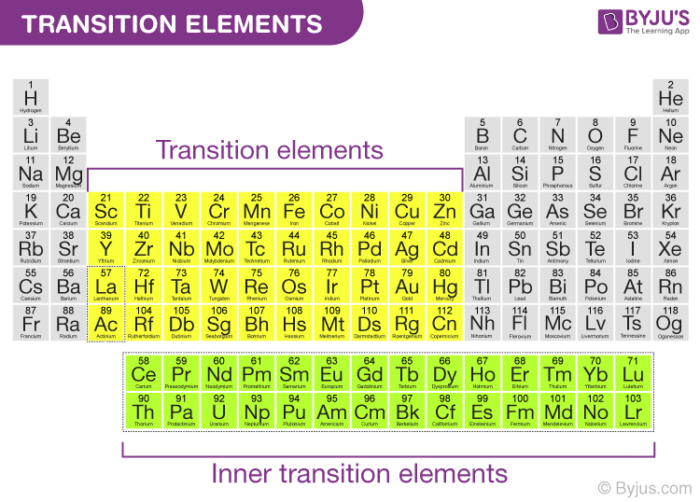 Source: byjus.com
Source: byjus.com
The transition metals exhibit typical metallic properties such as malleability ductility high tensile strength and metallic lustre. They are good conductors of heat and electricity they can be hammered or bent into shape easily. They occupy the middle portions of the long periods of the periodic table of the elements. Transition metal any of various chemical elements that have valence electrons i e electrons that can participate in the formation of chemical bonds in two shells instead of only one. For example the lanthanides all form stable 3 aqueous cations.
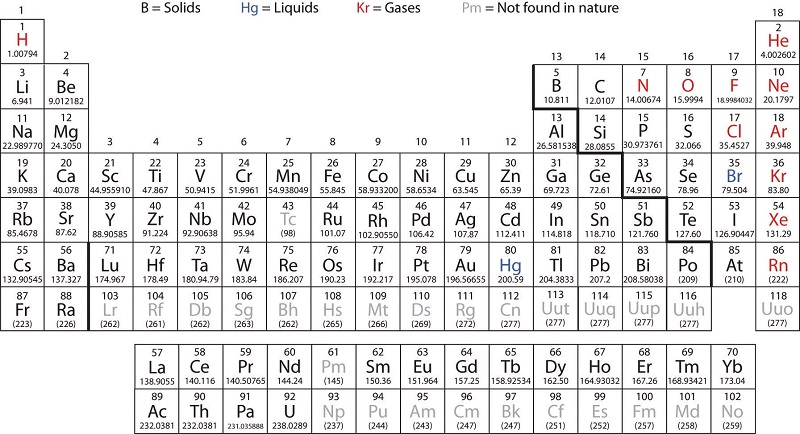 Source: chem.libretexts.org
Source: chem.libretexts.org
Transition metal any of various chemical elements that have valence electrons i e electrons that can participate in the formation of chemical bonds in two shells instead of only one. For example the lanthanides all form stable 3 aqueous cations. As can be seen from their reduction potentials table p1 some transition metals are strong reducing agents whereas others have very low reactivity. They are good conductors of heat and electricity they can be hammered or bent into shape easily. The transition metals have the following physical properties.
 Source: slideplayer.com
Source: slideplayer.com
Quick summary of the transition metal properties. Transition metals demonstrate a wide range of chemical behaviors. However trends can be observed in the metallic properties of the transition elements. Multiple oxidation states since there is a low energy gap between them. As can be seen from their reduction potentials table p1 some transition metals are strong reducing agents whereas others have very low reactivity.
Source: quora.com
They are good conductors of heat and electricity they can be hammered or bent into shape easily. For example the lanthanides all form stable 3 aqueous cations. The transition metals exhibit typical metallic properties such as malleability ductility high tensile strength and metallic lustre. As can be seen from their reduction potentials table p1 some transition metals are strong reducing agents whereas others have very low reactivity. They are good conductors of heat and electricity they can be hammered or bent into shape easily.
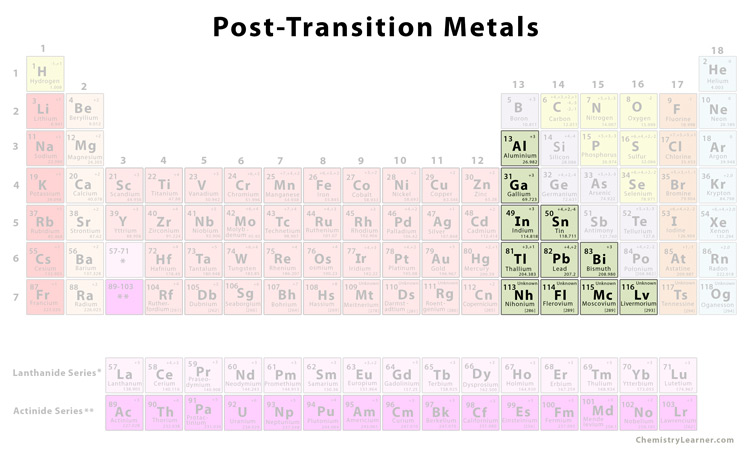 Source: chemistrylearner.com
Source: chemistrylearner.com
The transition metals exhibit typical metallic properties such as malleability ductility high tensile strength and metallic lustre. Transition metals demonstrate a wide range of chemical behaviors. For example the lanthanides all form stable 3 aqueous cations. Transition metal any of various chemical elements that have valence electrons i e electrons that can participate in the formation of chemical bonds in two shells instead of only one. However trends can be observed in the metallic properties of the transition elements.
 Source: slideplayer.com
Source: slideplayer.com
They occupy the middle portions of the long periods of the periodic table of the elements. However trends can be observed in the metallic properties of the transition elements. Transition metal any of various chemical elements that have valence electrons i e electrons that can participate in the formation of chemical bonds in two shells instead of only one. The transition metals exhibit typical metallic properties such as malleability ductility high tensile strength and metallic lustre. They are good conductors of heat and electricity they can be hammered or bent into shape easily.
 Source: wikihow.com
Source: wikihow.com
The transition metals exhibit typical metallic properties such as malleability ductility high tensile strength and metallic lustre. They are good conductors of heat and electricity they can be hammered or bent into shape easily. However trends can be observed in the metallic properties of the transition elements. They are generally good conductors of heat and electricity and tend to crystallize in bcc body centred cubic ccp cubic close packed or hcp hexagonally close packed structures. Quick summary of the transition metal properties.
 Source: slideshare.net
Source: slideshare.net
Transition metals demonstrate a wide range of chemical behaviors. For example the lanthanides all form stable 3 aqueous cations. They are generally good conductors of heat and electricity and tend to crystallize in bcc body centred cubic ccp cubic close packed or hcp hexagonally close packed structures. Multiple oxidation states since there is a low energy gap between them. They occupy the middle portions of the long periods of the periodic table of the elements.
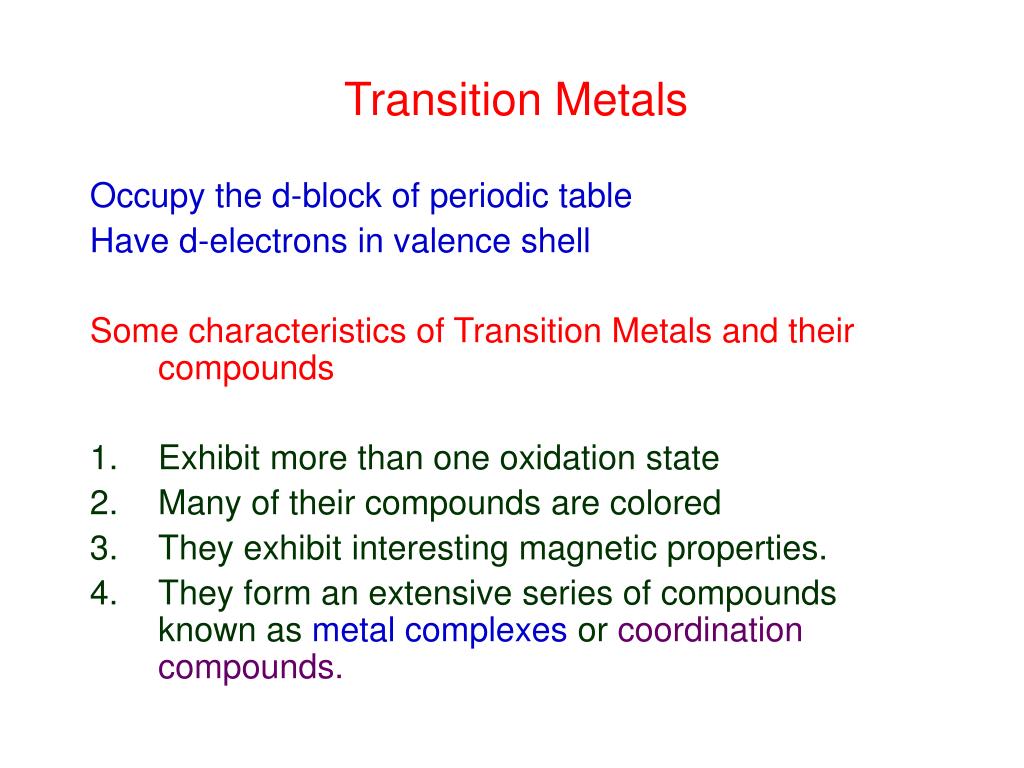 Source: slideserve.com
Source: slideserve.com
They occupy the middle portions of the long periods of the periodic table of the elements. They occupy the middle portions of the long periods of the periodic table of the elements. Multiple oxidation states since there is a low energy gap between them. Transition metals demonstrate a wide range of chemical behaviors. For example the lanthanides all form stable 3 aqueous cations.
 Source: toppr.com
Source: toppr.com
Quick summary of the transition metal properties. However trends can be observed in the metallic properties of the transition elements. Transition metals demonstrate a wide range of chemical behaviors. The transition metals exhibit typical metallic properties such as malleability ductility high tensile strength and metallic lustre. They are good conductors of heat and electricity they can be hammered or bent into shape easily.
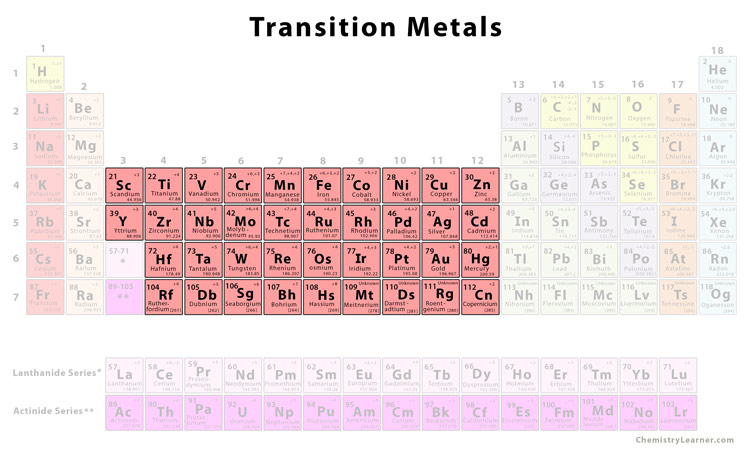 Source: chemistrylearner.com
Source: chemistrylearner.com
For example the lanthanides all form stable 3 aqueous cations. They occupy the middle portions of the long periods of the periodic table of the elements. They are good conductors of heat and electricity they can be hammered or bent into shape easily. They are generally good conductors of heat and electricity and tend to crystallize in bcc body centred cubic ccp cubic close packed or hcp hexagonally close packed structures. However trends can be observed in the metallic properties of the transition elements.
 Source: sciencenotes.org
Source: sciencenotes.org
Transition metal any of various chemical elements that have valence electrons i e electrons that can participate in the formation of chemical bonds in two shells instead of only one. The transition metals have the following physical properties. They occupy the middle portions of the long periods of the periodic table of the elements. They are generally good conductors of heat and electricity and tend to crystallize in bcc body centred cubic ccp cubic close packed or hcp hexagonally close packed structures. Multiple oxidation states since there is a low energy gap between them.
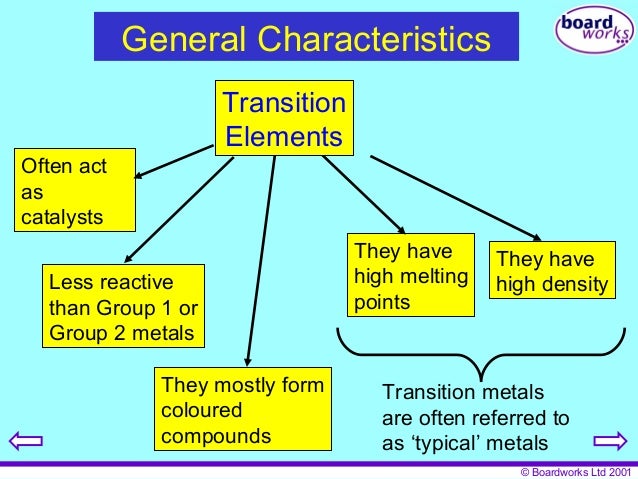 Source: slideshare.net
Source: slideshare.net
As can be seen from their reduction potentials table p1 some transition metals are strong reducing agents whereas others have very low reactivity. For example the lanthanides all form stable 3 aqueous cations. As can be seen from their reduction potentials table p1 some transition metals are strong reducing agents whereas others have very low reactivity. Transition metals demonstrate a wide range of chemical behaviors. They occupy the middle portions of the long periods of the periodic table of the elements.
 Source: britannica.com
Source: britannica.com
For example the lanthanides all form stable 3 aqueous cations. They are good conductors of heat and electricity they can be hammered or bent into shape easily. However trends can be observed in the metallic properties of the transition elements. They are generally good conductors of heat and electricity and tend to crystallize in bcc body centred cubic ccp cubic close packed or hcp hexagonally close packed structures. Multiple oxidation states since there is a low energy gap between them.
 Source: askiitians.com
Source: askiitians.com
They occupy the middle portions of the long periods of the periodic table of the elements. Transition metals demonstrate a wide range of chemical behaviors. As can be seen from their reduction potentials table p1 some transition metals are strong reducing agents whereas others have very low reactivity. Transition metal any of various chemical elements that have valence electrons i e electrons that can participate in the formation of chemical bonds in two shells instead of only one. They are good conductors of heat and electricity they can be hammered or bent into shape easily.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title transition metal characteristics by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.