The least reactive element
The Least Reactive Element. Similarly n can be considered as least reactive as compared to other member of the group. Nonmetals are elements that generally cannot conduct electricity. Neon is considered the most noble of the gases as it has the lowest reactivity in a group of elements with low reactivity. The least reactive elements are those who have a full outermost valence shell ie they have 8 electrons in the outer shell so elements such as helium neon radon or the transition elements.
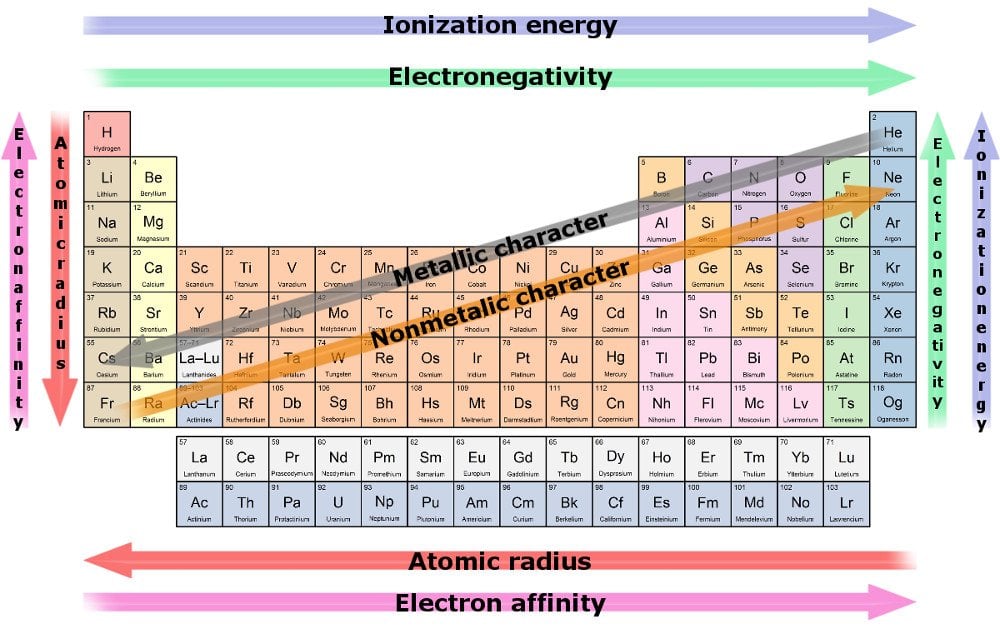 Which Is The Most Reactive Element In The Periodic Table Science Abc From scienceabc.com
Which Is The Most Reactive Element In The Periodic Table Science Abc From scienceabc.com
Technetium is the most reactive. Some nonmetals are very reactive whereas others are not reactive at all. Nonmetals are also poor conductors of heat and solid nonmetals are dull and brittle. In this manner which group of elements are the least reactive and why. Noble gases are the least reactive of all elements. Fluorine is the most reactive element of all in group 7.
Neon is the least reactive element on the periodic table.
In general the first member of group is least reactive as compared to all other member of the group. The noble gases group 18 on the far right are the least reactive of all elements because they have a full outer level of electrons. Neon is the least reactive element on the periodic table. In general the first member of group is least reactive as compared to all other member of the group. There is published data to help you determine relative. That s because they have eight valence electrons which fill their outer energy level.
Source: quora.com
In general the first member of group is least reactive as compared to all other member of the group. Neon is considered the most noble of the gases as it has the lowest reactivity in a group of elements with low reactivity. Most reactive na least ag34. Similarly n can be considered as least reactive as compared to other member of the group. In this manner which group of elements are the least reactive and why.
 Source: brainly.com
Source: brainly.com
This is followed by silver. Nonmetals are also poor conductors of heat and solid nonmetals are dull and brittle. There is published data to help you determine relative. Properties of nonmetals include a relatively low boiling point so many nonmetals are gases. Technetium is the most reactive.
 Source: toppr.com
Source: toppr.com
As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state. Nonmetals are also poor conductors of heat and solid nonmetals are dull and brittle. Noble gases are the least reactive of all elements. The least reactive elements are those who have a full outermost valence shell ie they have 8 electrons in the outer shell so elements such as helium neon radon or the transition elements. There is published data to help you determine relative.
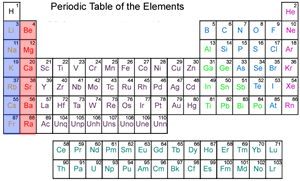 Source: dynamicscience.com.au
Source: dynamicscience.com.au
In this manner which group of elements are the least reactive and why. Neon has an atomic number of 10. The least reactive element in group 15 va of the periodic table share with your friends. The noble gases group 18 on the far right are the least reactive of all elements because they have a full outer level of electrons. As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state.
 Source: scienceabc.com
Source: scienceabc.com
Properties of nonmetals include a relatively low boiling point so many nonmetals are gases. Nonmetals are elements that generally cannot conduct electricity. In this manner which group of elements are the least reactive and why. As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state. Some nonmetals are very reactive whereas others are not reactive at all.
Source: quora.com
Noble gases are the least reactive of all elements. That s because they have eight valence electrons which fill their outer energy level. Neon has an atomic number of 10. Technetium is the most reactive. Neon is considered the most noble of the gases as it has the lowest reactivity in a group of elements with low reactivity.
 Source: quora.com
Source: quora.com
This is followed by silver. That s because they have eight valence electrons which fill their outer energy level. There is published data to help you determine relative. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. In general the first member of group is least reactive as compared to all other member of the group.
 Source: theperiodictablenick.weebly.com
Source: theperiodictablenick.weebly.com
Nonmetals are elements that generally cannot conduct electricity. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state. This is the most stable arrangement of electrons so noble gases rarely react with other elements and form compounds. This is followed by silver.
 Source: compoundchem.com
Source: compoundchem.com
Fluorine is the most reactive element of all in group 7. Nonmetals are also poor conductors of heat and solid nonmetals are dull and brittle. In general the first member of group is least reactive as compared to all other member of the group. Nonmetals are elements that generally cannot conduct electricity. That s because they have eight valence electrons which fill their outer energy level.
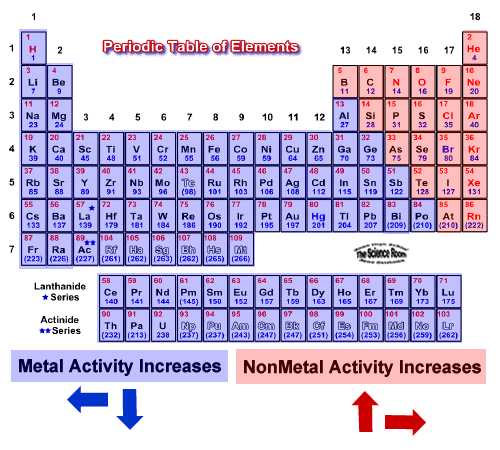 Source: socratic.org
Source: socratic.org
Noble gases are the least reactive of all elements. Some nonmetals are very reactive whereas others are not reactive at all. Nonmetals are elements that generally cannot conduct electricity. Neon is the least reactive element on the periodic table. This is followed by silver.
Source: quora.com
Fluorine is the most reactive element of all in group 7. Neon is the least reactive element on the periodic table. Some nonmetals are very reactive whereas others are not reactive at all. Nonmetals are elements that generally cannot conduct electricity. Noble gases are the least reactive of all elements.
 Source: pinterest.co.uk
Source: pinterest.co.uk
The noble gases group 18 on the far right are the least reactive of all elements because they have a full outer level of electrons. Neon is the least reactive element on the periodic table. There is published data to help you determine relative. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. Similarly n can be considered as least reactive as compared to other member of the group.
 Source: britannica.com
Source: britannica.com
The least reactive out of these three elements is strontium. Similarly n can be considered as least reactive as compared to other member of the group. There is published data to help you determine relative. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. Neon has an atomic number of 10.
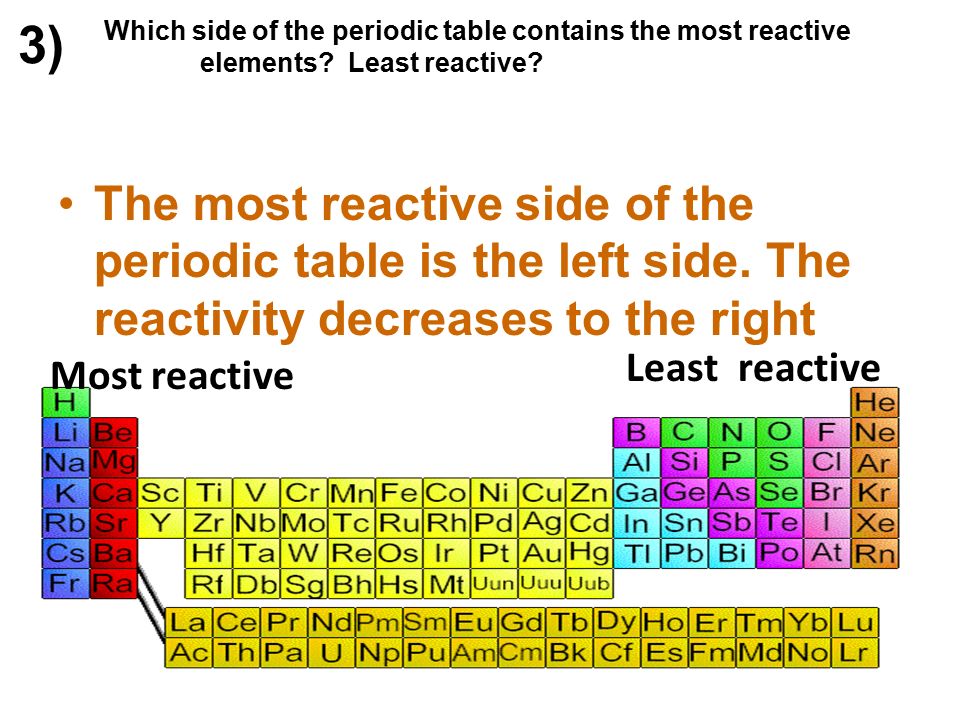 Source: slideplayer.com
Source: slideplayer.com
There is published data to help you determine relative. Noble gases are the least reactive of all elements. The most reactive metals such as sodium will react with cold water to produce hydrogen and the metal hydroxide. Most reactive na least ag34. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
Neon has an atomic number of 10. Neon is the least reactive element on the periodic table. 2 na s 2 h 2 o l 2 naoh aq h 2 g metals in the middle of the reactivity series such as iron will react with acids such as sulfuric acid but not water at normal temperatures to give hydrogen and a metal salt. As a noble gas neon is colorless and odorless with extremely low reactivity in its natural state. This is followed by silver.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title the least reactive element by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






