Stainless steel electrodes for electrolysis
Stainless Steel Electrodes For Electrolysis. Trivalent chromium and hexavalent chromium. If you water supply has iron ions in solution can t use stainless steel except as a getter. Over the past 40 years we have constantly modified and updated this important product in order to continually offer our clients the very latest technological developments. Pure water is non conductive so won t work.
 3d Printed Porous Electrodes For Advanced Electrochemical Flow Reactors A Ni Stainless Steel Electrode And Its Mass Transport Characteristics Sciencedirect From sciencedirect.com
3d Printed Porous Electrodes For Advanced Electrochemical Flow Reactors A Ni Stainless Steel Electrode And Its Mass Transport Characteristics Sciencedirect From sciencedirect.com
Pure water is non conductive so won t work. Stainless steel is indeed consumed when used in the electrolysis process although slowly. Would require some careful valving of course. Production of hexavalent chromium during electrolysis using a stainless steel as the cathode. The material has an excellent stability under dynamic long term test conditions and sustainable. Stainless steel does not visibly deteriorate during electrolysis but it slowly dissolves chromium into the electrolyte producing two chromium compounds.
Why you should not use stainless steel electrodes for electrolysis many people using the electrolysis method for rust reduction swear by stainless steel stating incorrectly that it s not consumed stays clean and seems safe.
Stable cell components and a good performance was achieved using raney nickel as a cathode and stainless steel 316l as an anode by means of a monopolar cell at 75 c which ran for one month at 300 ma cm 2. I know if i use stainless steel as the anode it does produce hexavalent chromium but what if it is a stainless cathode. Stainless steel electrodes the 312 or 29 9 type electrode is the leader of this group in popularity as well as physical properties. The important part is having a solvent mixed with the water. Trivalent chromium and hexavalent chromium. Stable cell components and a good performance was achieved using raney nickel as a cathode and stainless steel 316l as an anode by means of a monopolar cell at 75 c which ran for one month at 300 ma cm 2.
 Source: toppr.com
Source: toppr.com
You don t need specific metals for electrolysis just a metal that conducts electricity and the two electrodes don t need to be different metals as they do if you were creating an acid battery. Stainless steel is indeed consumed when used in the electrolysis process although slowly. The aisi 316l stainless steel demonstrates moderate catalytic activity toward both her and oer during electrochemical water splitting in electrolysis cell with anion exchange membrane. However if you use it as an anode in koh solutions and if the solution is hot 60c you will notice iron going into solution as a. Would require some careful valving of course.
 Source: scielo.br
Source: scielo.br
Stainless steel is indeed consumed when used in the electrolysis process although slowly. Pure water is non conductive so won t work. One suggestion might be say each hour rotate which electrode is and which is to reverse some of the chemistry that can set up at the anode. However if you use it as an anode in koh solutions and if the solution is hot 60c you will notice iron going into solution as a. Finally the proposed catalysts showed a total kinetic overpotential of about 550 mv at 75 c and 1 a cm 2.
 Source: sciencedirect.com
Source: sciencedirect.com
I know if i use stainless steel as the anode it does produce hexavalent chromium but what if it is a stainless cathode. I know if i use stainless steel as the anode it does produce hexavalent chromium but what if it is a stainless cathode. Stainless steel electrodes the 312 or 29 9 type electrode is the leader of this group in popularity as well as physical properties. However if you use it as an anode in koh solutions and if the solution is hot 60c you will notice iron going into solution as a. While trivalent chromium is relatively harmless hexavalent chromium is toxic carcinogenic and poisonous.
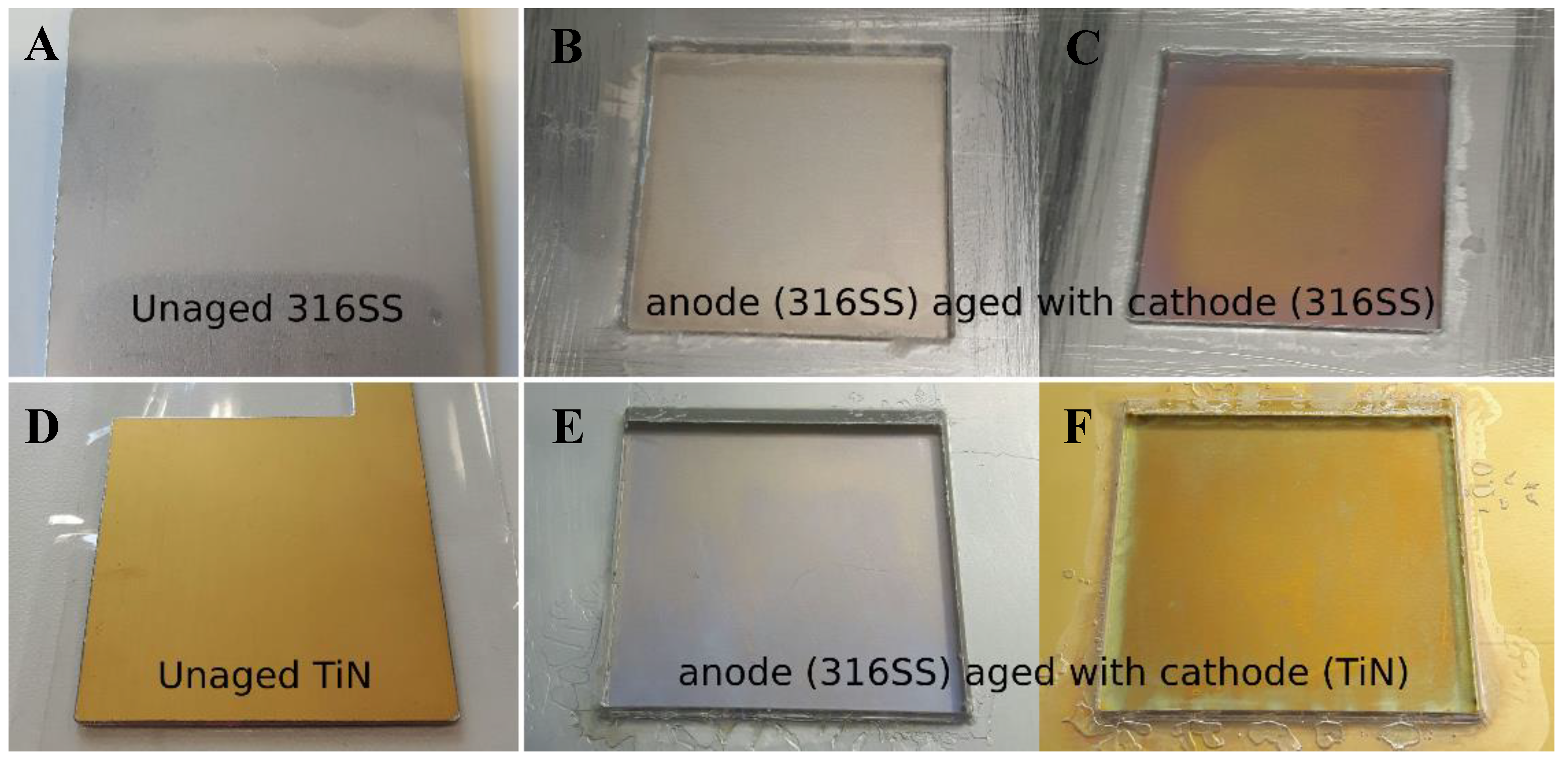 Source: mdpi.com
Source: mdpi.com
Trivalent chromium and hexavalent chromium. Why you should not use stainless steel electrodes for electrolysis many people using the electrolysis method for rust reduction swear by stainless steel stating incorrectly that it s not consumed stays clean and seems safe. Stainless steel can be used as a cathode for water electrolysis. Pure water is non conductive so won t work. Production of hexavalent chromium during electrolysis using a stainless steel as the cathode.
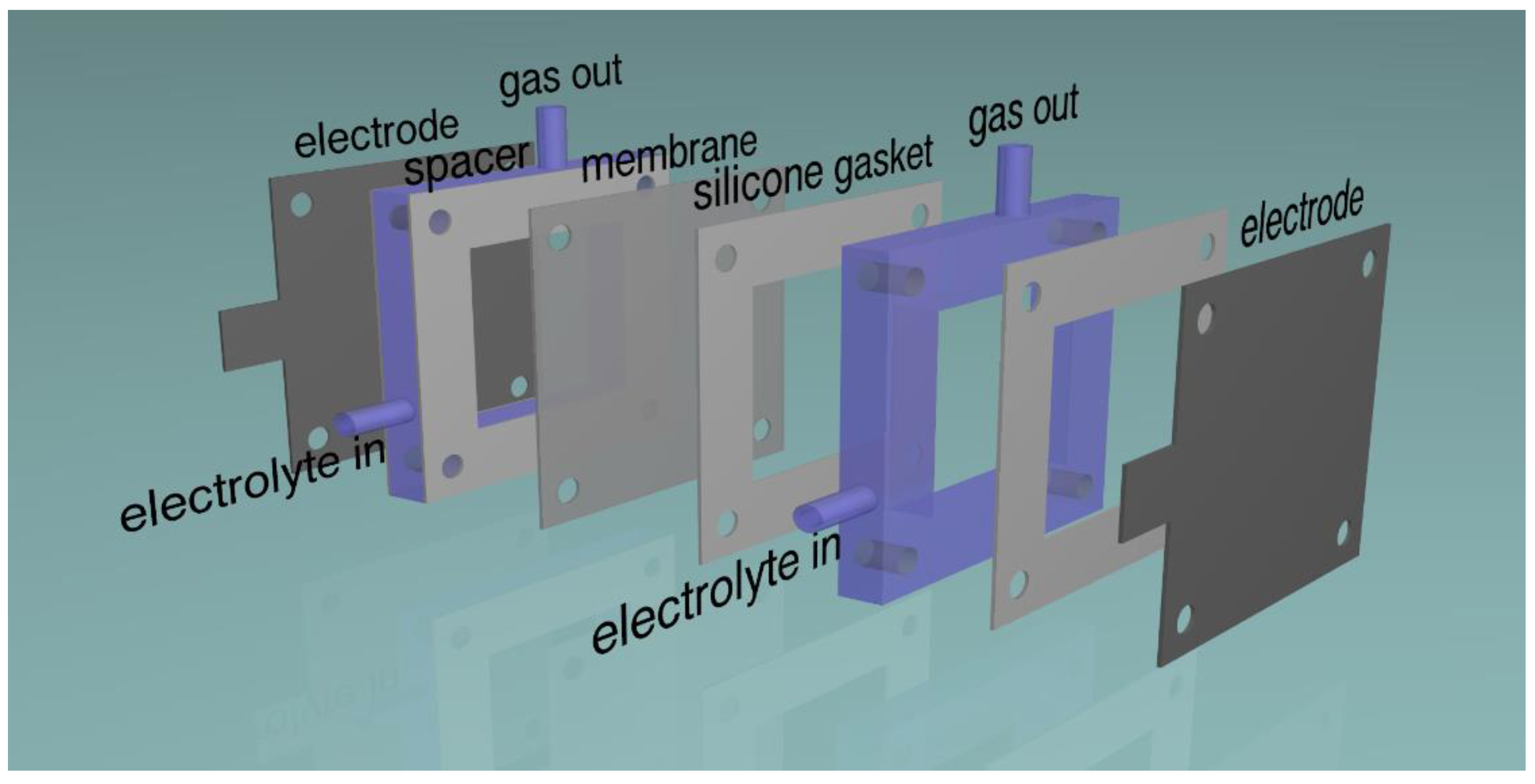 Source: mdpi.com
Source: mdpi.com
However if you use it as an anode in koh solutions and if the solution is hot 60c you will notice iron going into solution as a. However if you use it as an anode in koh solutions and if the solution is hot 60c you will notice iron going into solution as a. I know if i use stainless steel as the anode it does produce hexavalent chromium but what if it is a stainless cathode. Stainless steel electrodes the 312 or 29 9 type electrode is the leader of this group in popularity as well as physical properties. Finally the proposed catalysts showed a total kinetic overpotential of about 550 mv at 75 c and 1 a cm 2.
 Source: researchgate.net
Source: researchgate.net
The aisi 316l stainless steel demonstrates moderate catalytic activity toward both her and oer during electrochemical water splitting in electrolysis cell with anion exchange membrane. You don t need specific metals for electrolysis just a metal that conducts electricity and the two electrodes don t need to be different metals as they do if you were creating an acid battery. Trivalent chromium and hexavalent chromium. Finally the proposed catalysts showed a total kinetic overpotential of about 550 mv at 75 c and 1 a cm 2. However if you use it as an anode in koh solutions and if the solution is hot 60c you will notice iron going into solution as a.
 Source: x-mol.com
Source: x-mol.com
The important part is having a solvent mixed with the water. The material has an excellent stability under dynamic long term test conditions and sustainable. Ss 316 welding electrode. Stainless steel is indeed consumed when used in the electrolysis process although slowly. Chromium from electrode not oxidizing in electrolysis.
 Source: amazon.com
Source: amazon.com
One suggestion might be say each hour rotate which electrode is and which is to reverse some of the chemistry that can set up at the anode. Over the past 40 years we have constantly modified and updated this important product in order to continually offer our clients the very latest technological developments. Why you should not use stainless steel electrodes for electrolysis many people using the electrolysis method for rust reduction swear by stainless steel stating incorrectly that it s not consumed stays clean and seems safe. Finally the proposed catalysts showed a total kinetic overpotential of about 550 mv at 75 c and 1 a cm 2. If you water supply has iron ions in solution can t use stainless steel except as a getter.

Pure water is non conductive so won t work. Ask question asked 4 years. Ss 316 welding electrode. The important part is having a solvent mixed with the water. Trivalent chromium and hexavalent chromium.
 Source: nadascientific.com
Source: nadascientific.com
I know if i use stainless steel as the anode it does produce hexavalent chromium but what if it is a stainless cathode. Ask question asked 4 years. Would require some careful valving of course. While trivalent chromium is relatively harmless hexavalent chromium is toxic carcinogenic and poisonous. Trivalent chromium and hexavalent chromium.
 Source: metaldetectingworld.com
Source: metaldetectingworld.com
Ask question asked 4 years. Ask question asked 4 years. Pure water is non conductive so won t work. Chromium from electrode not oxidizing in electrolysis. One suggestion might be say each hour rotate which electrode is and which is to reverse some of the chemistry that can set up at the anode.
 Source: researchgate.net
Source: researchgate.net
The material has an excellent stability under dynamic long term test conditions and sustainable. One suggestion might be say each hour rotate which electrode is and which is to reverse some of the chemistry that can set up at the anode. While trivalent chromium is relatively harmless hexavalent chromium is toxic carcinogenic and poisonous. The important part is having a solvent mixed with the water. The aisi 316l stainless steel demonstrates moderate catalytic activity toward both her and oer during electrochemical water splitting in electrolysis cell with anion exchange membrane.
 Source: scielo.br
Source: scielo.br
Ask question asked 4 years. If you water supply has iron ions in solution can t use stainless steel except as a getter. Why you should not use stainless steel electrodes for electrolysis many people using the electrolysis method for rust reduction swear by stainless steel stating incorrectly that it s not consumed stays clean and seems safe. Trivalent chromium and hexavalent chromium. One suggestion might be say each hour rotate which electrode is and which is to reverse some of the chemistry that can set up at the anode.
 Source: homesciencetools.com
Source: homesciencetools.com
Stainless steel is indeed consumed when used in the electrolysis process although slowly. The aisi 316l stainless steel demonstrates moderate catalytic activity toward both her and oer during electrochemical water splitting in electrolysis cell with anion exchange membrane. Would require some careful valving of course. The material has an excellent stability under dynamic long term test conditions and sustainable. Stainless steel electrodes the 312 or 29 9 type electrode is the leader of this group in popularity as well as physical properties.
 Source: sciencedirect.com
Source: sciencedirect.com
One suggestion might be say each hour rotate which electrode is and which is to reverse some of the chemistry that can set up at the anode. Chromium from electrode not oxidizing in electrolysis. One suggestion might be say each hour rotate which electrode is and which is to reverse some of the chemistry that can set up at the anode. The important part is having a solvent mixed with the water. Stainless steel can be used as a cathode for water electrolysis.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title stainless steel electrodes for electrolysis by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





