Rate of electrolysis of water
Rate Of Electrolysis Of Water. Typically a water electrolysis unit consists of an anode a cathode separated with an electrolyte and a power supply. This change in internal energy must be accompanied by the expansion of the gases produced so the change in enthalpy represents the necessary energy to accomplish the electrolysis. Increased surface area of electrodes. Electrolysis of water will begin around a minimum of 1 2 volts and will increase in rate as the voltage is increased.
 Pdf An Experimental Study On The Effect Of Electrolytic Concentration On The Rate Of Hydrogen Production From researchgate.net
Pdf An Experimental Study On The Effect Of Electrolytic Concentration On The Rate Of Hydrogen Production From researchgate.net
Typically a water electrolysis unit consists of an anode a cathode separated with an electrolyte and a power supply. Electrolysis of water will begin around a minimum of 1 2 volts and will increase in rate as the voltage is increased. The average energy consumption for internal compression is around 3. The net reaction of electrolysis of water is given as. 2h 2 o l 2h 2 g o 2 g e 1 24 v. 2h 2 o l 2e h 2 g 2oh e 0 42 v.
Half reactions in the electrolysis of pure water at ph 7 and at 25 care at cathode.
Water electrolysis is the process whereby water is split into hydrogen and oxygen through the application of electrical energy as in eq. Increased rate of electrolysis. The net reaction of electrolysis of water is given as. 2h 2 o o 2 g 4h 4e e 0 82 v. The average energy consumption for internal compression is around 3. δu δh pδv 285 83 kj 3 72 kj 282 1 kj.
 Source: scielo.br
Source: scielo.br
2h 2 o l 2h 2 g o 2 g e 1 24 v. Increased rate of electrolysis. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated. Increased concentration of ions in electrolyte. Efficiency is key i ve read that electrolysis is most efficient between 1 24 and 1 48v depending on who you ask.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Efficiency is key i ve read that electrolysis is most efficient between 1 24 and 1 48v depending on who you ask. Half reactions in the electrolysis of pure water at ph 7 and at 25 care at cathode. Electrolysis begins at 1 229v and it s the current that largely determines the amounts of hydrogen and oxygen formed. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated. Efficiency is key i ve read that electrolysis is most efficient between 1 24 and 1 48v depending on who you ask.
 Source: sciencedirect.com
Source: sciencedirect.com
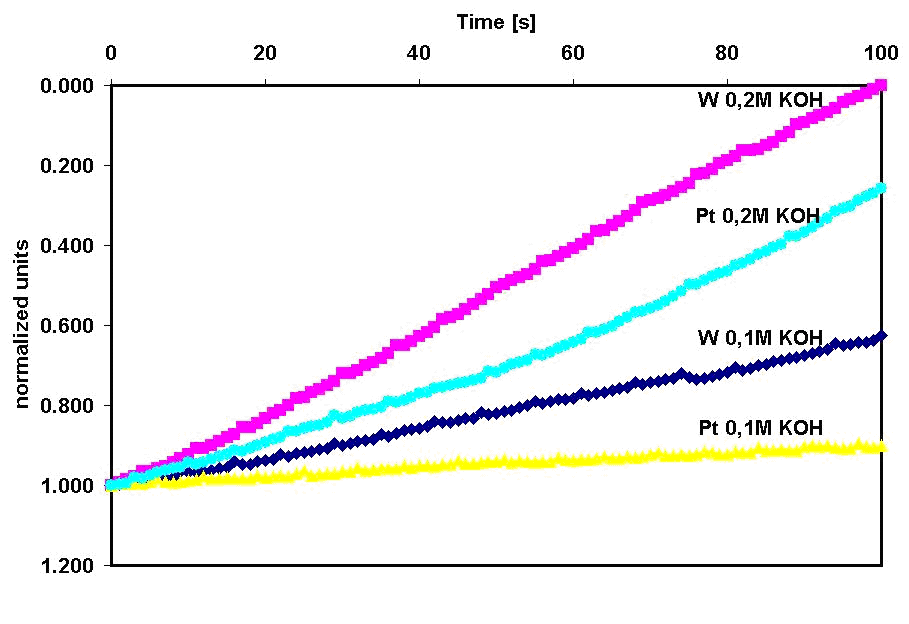
High pressure electrolysis is the electrolysis of water with a compressed hydrogen output around 12 20 mpa 120 200 bar 1740 2900 psi. Water electrolysis was done in specially constructed electrolytic cell for water treatment under dynamic conditions with the flow rate of 0 01 m s fig. Efficiency is key i ve read that electrolysis is most efficient between 1 24 and 1 48v depending on who you ask. Electrolysis of water will begin around a minimum of 1 2 volts and will increase in rate as the voltage is increased. Typically the electrolysis is carried out around 6 volts.
 Source: intechopen.com
Source: intechopen.com
Increased current and therefore increased rate of electrolysis. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated. Increased rate of electrolysis. Typically a water electrolysis unit consists of an anode a cathode separated with an electrolyte and a power supply. Increased surface area of electrodes.
 Source: researchgate.net
Source: researchgate.net
2h 2 o l 2e h 2 g 2oh e 0 42 v. The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. The net reaction of electrolysis of water is given as. Increased surface area of electrodes. δu δh pδv 285 83 kj 3 72 kj 282 1 kj.
 Source: intechopen.com
Source: intechopen.com
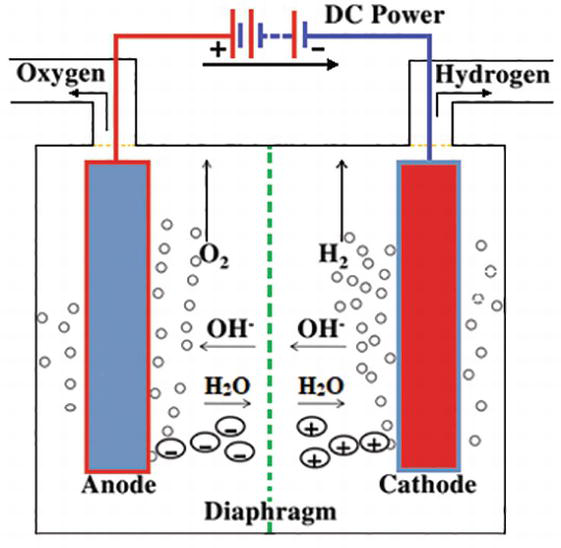
This change in internal energy must be accompanied by the expansion of the gases produced so the change in enthalpy represents the necessary energy to accomplish the electrolysis. 2h 2 o l 2h 2 g o 2 g e 1 24 v. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as ce h 2so 4 has been added. Typically a water electrolysis unit consists of an anode a cathode separated with an electrolyte and a power supply. Increased current and therefore increased rate of electrolysis.
 Source: scielo.br
Source: scielo.br
The electrolysis of water produces hydrogen and oxygen gases. The net reaction of electrolysis of water is given as. The average energy consumption for internal compression is around 3. The electrolysis of water produces hydrogen and oxygen gases. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated.
 Source: researchgate.net
Source: researchgate.net
Increased current and therefore increased rate of electrolysis. Increased current and therefore increased rate of electrolysis. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated. 2h 2 o l 2h 2 g o 2 g e 1 24 v. Half reactions in the electrolysis of pure water at ph 7 and at 25 care at cathode.
 Source: carboncommentary.com
Source: carboncommentary.com
Half reactions in the electrolysis of pure water at ph 7 and at 25 care at cathode. δu δh pδv 285 83 kj 3 72 kj 282 1 kj. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated. Increased current and therefore increased rate of electrolysis. Increased surface area of electrodes.
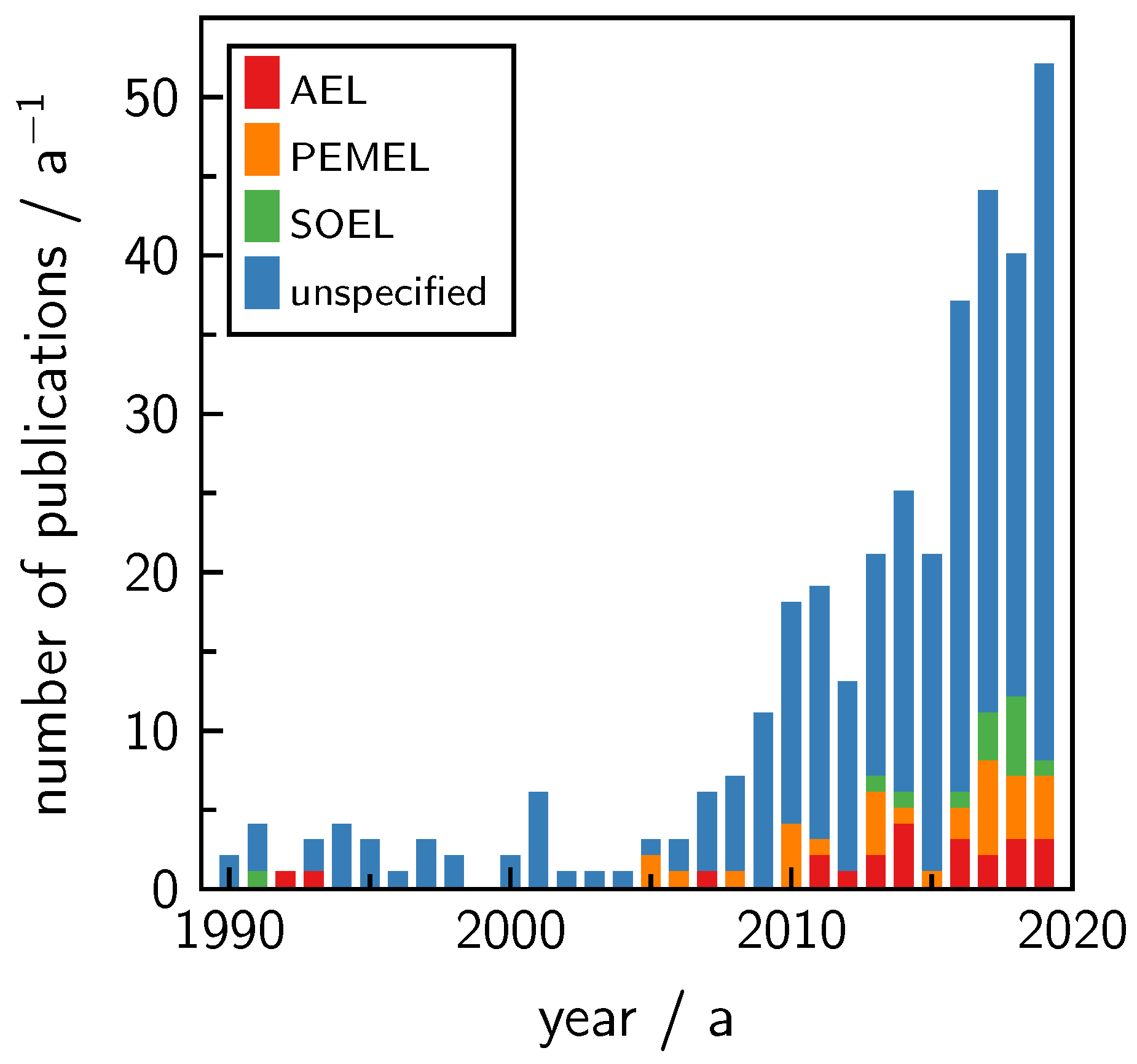 Source: mdpi.com
Source: mdpi.com
Typically a water electrolysis unit consists of an anode a cathode separated with an electrolyte and a power supply. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as ce h 2so 4 has been added. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated. Typically the electrolysis is carried out around 6 volts. Water electrolysis is the process whereby water is split into hydrogen and oxygen through the application of electrical energy as in eq.
 Source: scielo.br
Source: scielo.br
Increased current and therefore increased rate of electrolysis. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated. The electrolysis of water produces hydrogen and oxygen gases. Typically the electrolysis is carried out around 6 volts. Electrolysis of water cell potential and thermodynamic feasibility.
 Source: sciencedirect.com
Source: sciencedirect.com
However it is not necessary to put in this whole amount in the form of electrical energy. Typically a water electrolysis unit consists of an anode a cathode separated with an electrolyte and a power supply. That may seem counter intuitive but v ir really works. Though the conductivity of the electrolyte and the energy efficiency of water electrolysis increase as the temperature increases the present day water electrolysis cells are usually operated at 60 80 c for the caustic potash cell and at 50 70 c for the caustic soda cell respectively in order to reduce the consumption of electrolyzer materials. The electrolysis of water produces hydrogen and oxygen gases.
 Source: intechopen.com
Source: intechopen.com
By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated. Water electrolysis is the process whereby water is split into hydrogen and oxygen through the application of electrical energy as in eq. Typically a water electrolysis unit consists of an anode a cathode separated with an electrolyte and a power supply. High pressure electrolysis is the electrolysis of water with a compressed hydrogen output around 12 20 mpa 120 200 bar 1740 2900 psi. 2h 2 o o 2 g 4h 4e e 0 82 v.
 Source: researchgate.net
Source: researchgate.net
Water electrolysis was done in specially constructed electrolytic cell for water treatment under dynamic conditions with the flow rate of 0 01 m s fig. Increased current and therefore increased rate of electrolysis. Electrolysis of water cell potential and thermodynamic feasibility. The electrolytic cell consists of a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as ce h 2so 4 has been added. Increased rate of electrolysis.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Increased rate of electrolysis. The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. The net reaction of electrolysis of water is given as. Electrolysis of water cell potential and thermodynamic feasibility. By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor is eliminated.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title rate of electrolysis of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.