Pure substance vs mixtures
Pure Substance Vs Mixtures. Pure substances have specific melting and boiling points. The physical and chemical properties of pure substances are non changing if it is on its own without disturbing. All these substances fall into two major categories. The word pure is used in chemistry in a different way from its everyday meaning.
 Compound Vs Mixture Difference And Comparison Diffen From diffen.com
Compound Vs Mixture Difference And Comparison Diffen From diffen.com
A mixture is made up of a combination of two or more substances that are not united using a chemical reaction. Mixture is usually described as a substance that is made up of the combination of two or more substances in different proportions. We come across a lot of substances such as water fuel food beverages and medicine during our daily activities. Although chemists have a difficult time separating compounds into their specific elements the different parts of a mixture can be easily separated by physical means such as filtration. For example shops sell cartons labelled as pure orange juice. Pure substances and mixtures the meaning of pure.
It is possible to separate mixtures by evaporation magnetic separation etc.
All these substances fall into two major categories. Purity pure substances are of course pure. They are either pure substances or mixtures. Main difference pure substance vs mixture. The word pure is used in chemistry in a different way from its everyday meaning. We come across a lot of substances such as water fuel food beverages and medicine during our daily activities.
 Source: diffen.com
Source: diffen.com
Pure substances have specific melting and boiling points. The main difference between pure substance and mixture lies in their composition. All these substances fall into two major categories. The graphs below show the cooling curves for a pure sample of a compound called salol. Mixtures melt and boil over a range of temperatures.
 Source: ashleygolbuschemistry.weebly.com
Source: ashleygolbuschemistry.weebly.com
This section aims at reviewing and evaluating newly acquired knowle. Main difference pure substance vs mixture. The basic difference between pure substance and mixture is that pure substances are always composed of the same kind of particles and are homogeneous in nature with respect of origin while mixtures are variably composed having components retains its characteristic properties. A pure substance is often described as substances that are made up of only one type of atom or molecule. Separation it is not possible to separate pure substances.
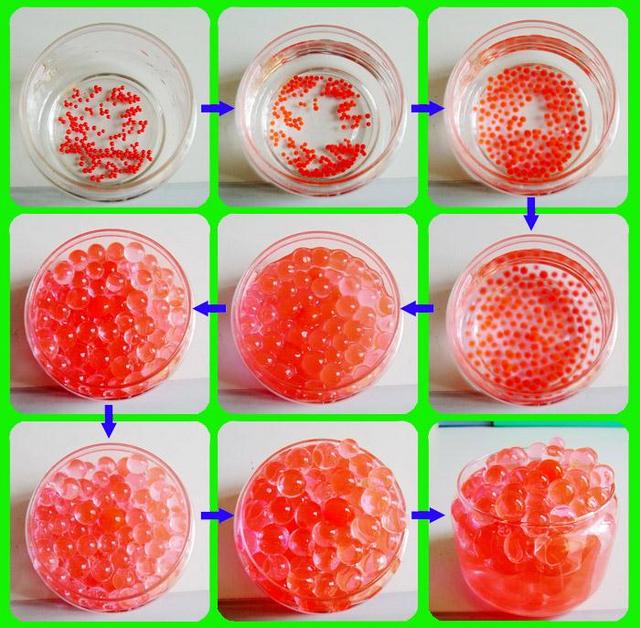
 Source: nedssurvivalguide.weebly.com
Source: nedssurvivalguide.weebly.com
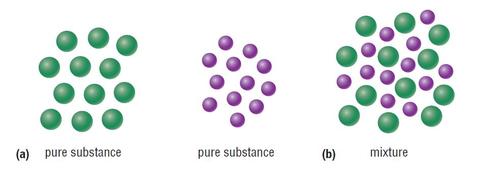
Mixtures are physical combinations of pure substances that have no definite or constant composition the composition of a mixture varies according to who prepares the mixture. Although chemists have a difficult time separating compounds into their specific elements the different parts of a mixture can be easily separated by physical means such as filtration. The main difference between pure substance and mixture lies in their composition. Chemical properties of pure substances are constant and definite too. Pure substances have specific melting and boiling points.
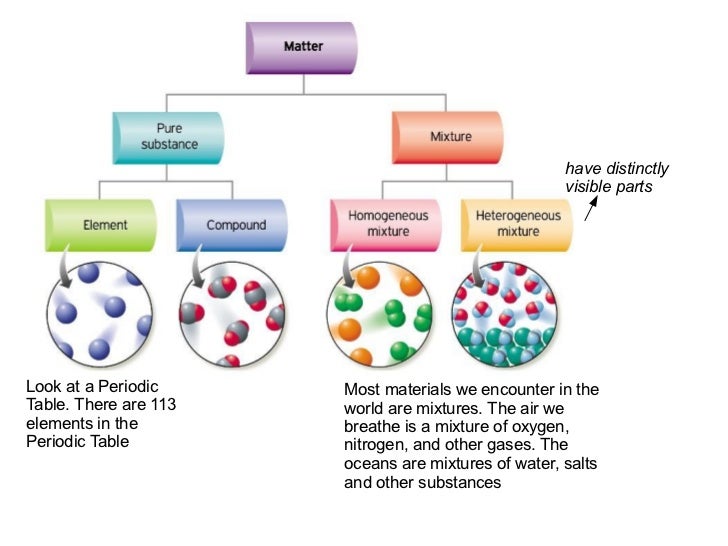 Source: pt.slideshare.net
Source: pt.slideshare.net
Mixtures have variable or indefinite chemical properties. Although chemists have a difficult time separating compounds into their specific elements the different parts of a mixture can be easily separated by physical means such as filtration. For example shops sell cartons labelled as pure orange juice. The physical and chemical properties of pure substances are non changing if it is on its own without disturbing. Mixtures melt and boil over a range of temperatures.
Source: docs.google.com
For example shops sell cartons labelled as pure orange juice. The main difference between pure substance and mixture lies in their composition. It is possible to separate mixtures by evaporation magnetic separation etc. A mixture is made up of a combination of two or more substances that are not united using a chemical reaction. Mixture is usually described as a substance that is made up of the combination of two or more substances in different proportions.
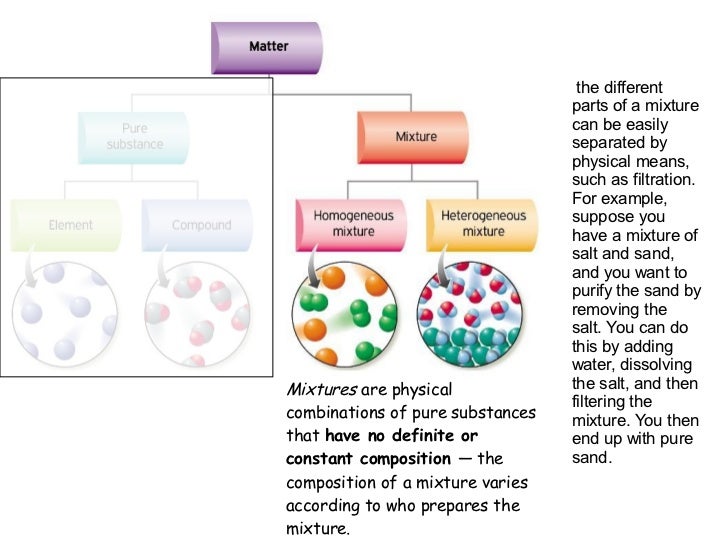 Source: pt.slideshare.net
Source: pt.slideshare.net
A mixture is made up of a combination of two or more substances that are not united using a chemical reaction. A pure substance is matter which cannot be separated into its basic components by using a physical or a chemical process. The basic difference between pure substance and mixture is that pure substances are always composed of the same kind of particles and are homogeneous in nature with respect of origin while mixtures are variably composed having components retains its characteristic properties. Although chemists have a difficult time separating compounds into their specific elements the different parts of a mixture can be easily separated by physical means such as filtration. Chemical properties of pure substances are constant and definite too.
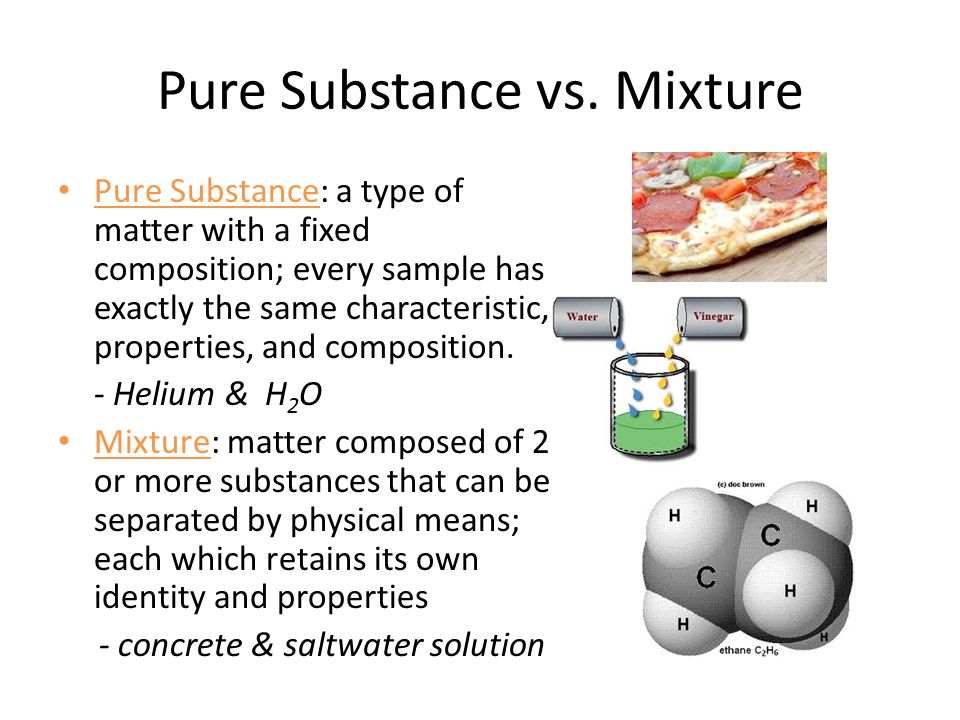 Source: slideplayer.com
Source: slideplayer.com
Mixtures melt and boil over a range of temperatures. Main difference pure substance vs mixture. Chemical properties of pure substances are constant and definite too. Pure substances and mixtures the meaning of pure. The main difference between pure substance and mixture lies in their composition.
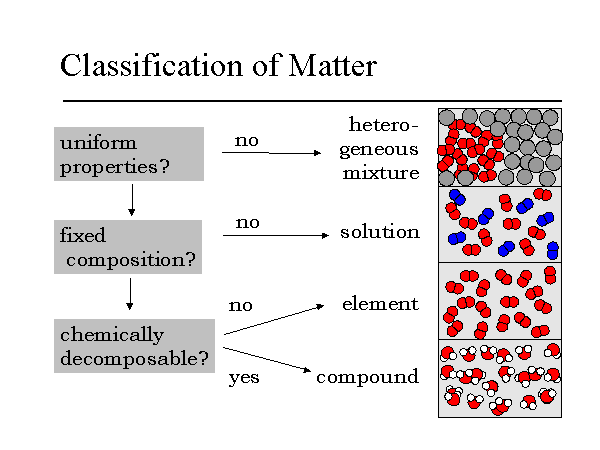 Source: chemsite.lsrhs.net
Source: chemsite.lsrhs.net
Pure substances have specific melting and boiling points. A pure substance is often described as substances that are made up of only one type of atom or molecule. The basic difference between pure substance and mixture is that pure substances are always composed of the same kind of particles and are homogeneous in nature with respect of origin while mixtures are variably composed having components retains its characteristic properties. We come across a lot of substances such as water fuel food beverages and medicine during our daily activities. Mixture a pure substance is made up of the same kind of molecules whereas mixture is made up of two different molecules.
 Source: slideshare.net
Source: slideshare.net
Mixture is usually described as a substance that is made up of the combination of two or more substances in different proportions. They are either pure substances or mixtures. All these substances fall into two major categories. Mixture a pure substance is made up of the same kind of molecules whereas mixture is made up of two different molecules. A mixture is made up of a combination of two or more substances that are not united using a chemical reaction.
 Source: toppr.com
Source: toppr.com
Although chemists have a difficult time separating compounds into their specific elements the different parts of a mixture can be easily separated by physical means such as filtration. For example shops sell cartons labelled as pure orange juice. Separation it is not possible to separate pure substances. Pure substances and mixtures the meaning of pure. We come across a lot of substances such as water fuel food beverages and medicine during our daily activities.
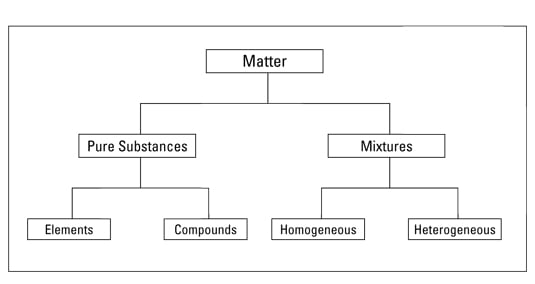 Source: dummies.com
Source: dummies.com
A pure substance is often described as substances that are made up of only one type of atom or molecule. Mixture is usually described as a substance that is made up of the combination of two or more substances in different proportions. Pure substances and mixtures the meaning of pure. Although chemists have a difficult time separating compounds into their specific elements the different parts of a mixture can be easily separated by physical means such as filtration. Purity pure substances are of course pure.
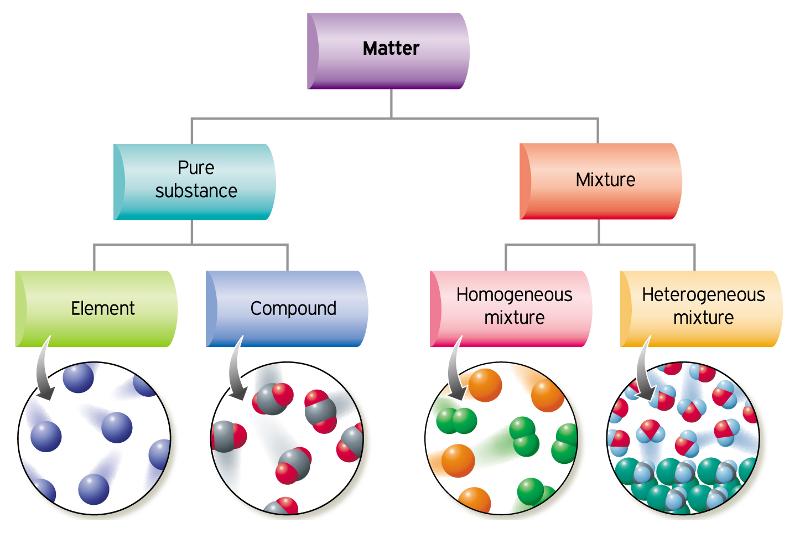 Source: winkelhage.com
Source: winkelhage.com
The basic difference between pure substance and mixture is that pure substances are always composed of the same kind of particles and are homogeneous in nature with respect of origin while mixtures are variably composed having components retains its characteristic properties. It is possible to separate mixtures by evaporation magnetic separation etc. The main difference between pure substance and mixture lies in their composition. Here s a video from the evaluate section in the pure substances and mixtures 5e lesson. All these substances fall into two major categories.
 Source: bioprofe.com
Source: bioprofe.com
Mixture a pure substance is made up of the same kind of molecules whereas mixture is made up of two different molecules. The main difference between pure substance and mixture lies in their composition. Mixture is usually described as a substance that is made up of the combination of two or more substances in different proportions. Mixture a pure substance is made up of the same kind of molecules whereas mixture is made up of two different molecules. A pure substance is matter which cannot be separated into its basic components by using a physical or a chemical process.
 Source: quizlet.com
Source: quizlet.com
Main difference pure substance vs mixture. A pure substance is matter which cannot be separated into its basic components by using a physical or a chemical process. Mixture is usually described as a substance that is made up of the combination of two or more substances in different proportions. Here s a video from the evaluate section in the pure substances and mixtures 5e lesson. For example shops sell cartons labelled as pure orange juice.
 Source: chem.libretexts.org
Source: chem.libretexts.org
A pure substance is often described as substances that are made up of only one type of atom or molecule. The main difference between pure substance and mixture lies in their composition. Mixtures have variable or indefinite chemical properties. Main difference pure substance vs mixture. A pure substance is matter which cannot be separated into its basic components by using a physical or a chemical process.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title pure substance vs mixtures by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.