Properties of pure metals
Properties Of Pure Metals. Titanium cobalt nickel copper zinc zirconium molybdenum tin and lead have been tested for their antibacterial properties against two bacterial strains gram positive staphylococcus aureus and gram negative escherichia coli. Commercially pure metals are 99 pure minimum. The most important electrical properties of metals are conductivity resistivity and dielectric strength both links are external. Pure metals are too soft and malleable.
Are Alloys Usually Denser Than Pure Metals Quora From quora.com
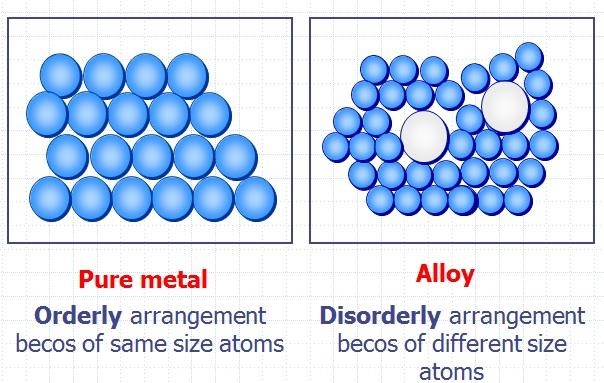
Pure metals have many useful properties but they are not widely used because of two main reasons. This was accomplished using two assay methods the film contact method and the shaking flask method. High melting and boiling points. Pure metals may react with air and water i e. A pure metal is a substance that contains atoms of only one type of metallic element such as aluminum gold copper lead or zinc. An alloy is defined as a mixture of a metal with at least one other element.
This was accomplished using two assay methods the film contact method and the shaking flask method.
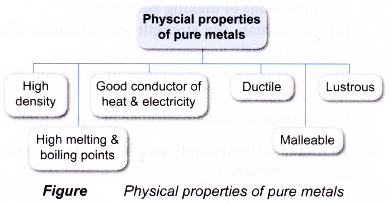
The handbook of pure metal properties consists of three interrelated parts. Good conductor of heat and electricity. In this study nine pure metals viz. As such we often use alloys instead of the pure metals. Metalmen distributes a wide range of pure metals for all your application requirements. Malleable can be beaten into thin sheets without cracking.
 Source: researchgate.net
Source: researchgate.net
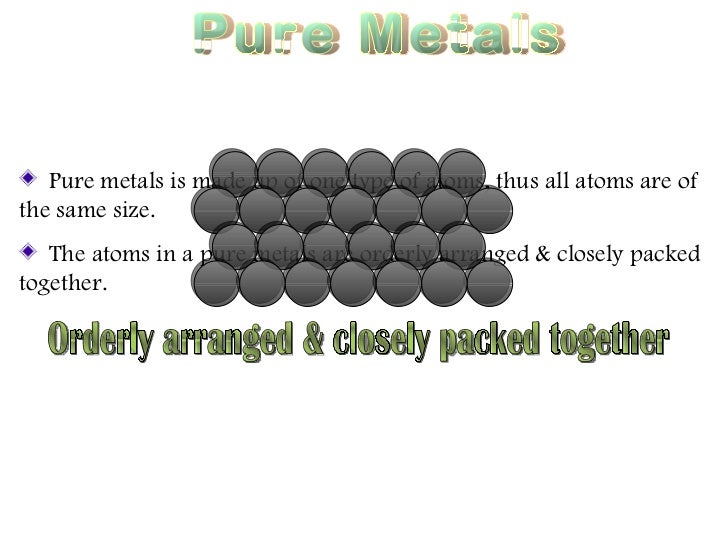
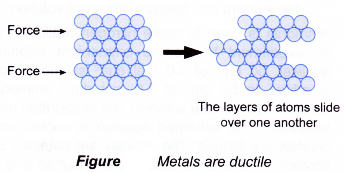
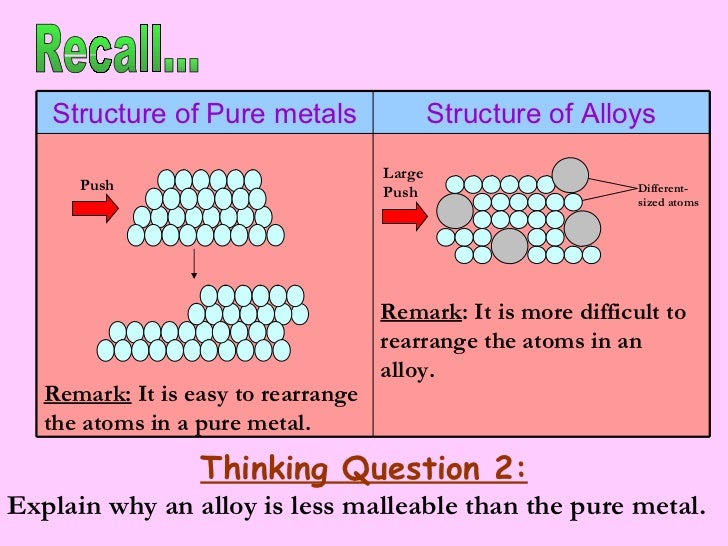
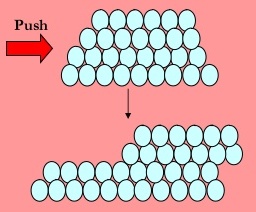
In this study nine pure metals viz. Some of the metals listed below are available as commercially pure and many can be manufactured to be extremely pure often 99 999 minimum referred to as five nines min. In pure metals the atoms are arranged in neat layers and when a force is applied to the metal eg by being hit with a hammer the layers of metal atoms can slide over each other giving the. An alloy is defined as a mixture of a metal with at least one other element. Pure metals are too soft and malleable.
 Source: simplechemconcepts.com
Source: simplechemconcepts.com
The most important electrical properties of metals are conductivity resistivity and dielectric strength both links are external. 44 pure metals including the s valence alkali p valence groups iii to v and d valence transition metals were selected. The most important electrical properties of metals are conductivity resistivity and dielectric strength both links are external. Pure metals are too soft and malleable. Lustrous becomes shiny when polished.
 Source: slideshare.net
Source: slideshare.net
Some of the metals listed below are available as commercially pure and many can be manufactured to be extremely pure often 99 999 minimum referred to as five nines min. As such we often use alloys instead of the pure metals. Metalmen distributes a wide range of pure metals for all your application requirements. Titanium cobalt nickel copper zinc zirconium molybdenum tin and lead have been tested for their antibacterial properties against two bacterial strains gram positive staphylococcus aureus and gram negative escherichia coli. Summary tables for description and comparison of various properties of pure metals effects of some factors on the mechanical properties of pure metals and properties of all metallic elements in the sequence of subgroups of.
 Source: slideshare.net
Source: slideshare.net
Pure metals are those metals that have not been alloyed with other metallic elements. Some of the metals listed below are available as commercially pure and many can be manufactured to be extremely pure often 99 999 minimum referred to as five nines min. Good conductor of heat and electricity. In this study nine pure metals viz. This was accomplished using two assay methods the film contact method and the shaking flask method.
 Source: aplustopper.com
Source: aplustopper.com
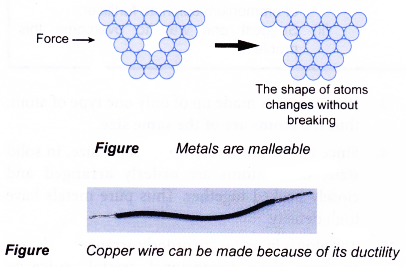
These properties of pure metals are reflected by their arrangement of atoms. This was accomplished using two assay methods the film contact method and the shaking flask method. Typical pure metals have the following physical properties. Ductile can be drawn into fine wire. Metalmen distributes a wide range of pure metals for all your application requirements.
 Source: aplustopper.com
Source: aplustopper.com
High melting and boiling points. Summary tables for description and comparison of various properties of pure metals effects of some factors on the mechanical properties of pure metals and properties of all metallic elements in the sequence of subgroups of. This was accomplished using two assay methods the film contact method and the shaking flask method. Commercially pure metals are 99 pure minimum. Metalmen distributes a wide range of pure metals for all your application requirements.
 Source: slideshare.net
Source: slideshare.net
High melting and boiling points. Good conductor of heat and electricity. The most important electrical properties of metals are conductivity resistivity and dielectric strength both links are external. This article presents the following characteristics of pure metals. Ductile can be drawn into fine wire.
Source: quora.com
High melting and boiling points. Some of the metals listed below are available as commercially pure and many can be manufactured to be extremely pure often 99 999 minimum referred to as five nines min. An alloy is defined as a mixture of a metal with at least one other element. Commercially pure metals are 99 pure minimum. First principles calculations were carried out to study the equilibrium properties of metals including the electrons at bonding critical point.
 Source: researchgate.net
Source: researchgate.net
Metalmen distributes a wide range of pure metals for all your application requirements. The handbook of pure metal properties consists of three interrelated parts. First principles calculations were carried out to study the equilibrium properties of metals including the electrons at bonding critical point. The most important electrical properties of metals are conductivity resistivity and dielectric strength both links are external. Lustrous becomes shiny when polished.
 Source: simplechemconcepts.com
Source: simplechemconcepts.com
Malleable can be beaten into thin sheets without cracking. Structure chemical composition mass characteristics thermal properties electrical properties chemical properties magnetic properties optical properties fabrication characteristics nuclear properties and mechanical properties. Summary tables for description and comparison of various properties of pure metals effects of some factors on the mechanical properties of pure metals and properties of all metallic elements in the sequence of subgroups of. Pure metals are those metals that have not been alloyed with other metallic elements. This was accomplished using two assay methods the film contact method and the shaking flask method.
 Source: slideshare.net
Source: slideshare.net
These properties of pure metals are reflected by their arrangement of atoms. Pure metals are too soft and malleable. Metalmen distributes a wide range of pure metals for all your application requirements. Most metals very rarely if ever appear in their pure form in nature and instead must be extracted from a metal ore. Pure metals have many useful properties but they are not widely used because of two main reasons.
 Source: aplustopper.com
Source: aplustopper.com
High melting and boiling points. These properties of pure metals are reflected by their arrangement of atoms. Most metals very rarely if ever appear in their pure form in nature and instead must be extracted from a metal ore. First principles calculations were carried out to study the equilibrium properties of metals including the electrons at bonding critical point. Pure metals have many useful properties but they are not widely used because of two main reasons.
 Source: aplustopper.com
Source: aplustopper.com
Titanium cobalt nickel copper zinc zirconium molybdenum tin and lead have been tested for their antibacterial properties against two bacterial strains gram positive staphylococcus aureus and gram negative escherichia coli. 44 pure metals including the s valence alkali p valence groups iii to v and d valence transition metals were selected. Typical pure metals have the following physical properties. A pure metal is a substance that contains atoms of only one type of metallic element such as aluminum gold copper lead or zinc. This was accomplished using two assay methods the film contact method and the shaking flask method.
 Source: 4mechtech.blogspot.com
Source: 4mechtech.blogspot.com
This was accomplished using two assay methods the film contact method and the shaking flask method. As such we often use alloys instead of the pure metals. In pure metals the atoms are arranged in neat layers and when a force is applied to the metal eg by being hit with a hammer the layers of metal atoms can slide over each other giving the. Ductile can be drawn into fine wire. This article presents the following characteristics of pure metals.
 Source: joannewong.weebly.com
Source: joannewong.weebly.com
The most important electrical properties of metals are conductivity resistivity and dielectric strength both links are external. High melting and boiling points. A pure metal is a substance that contains atoms of only one type of metallic element such as aluminum gold copper lead or zinc. Pure metals are those metals that have not been alloyed with other metallic elements. Pure metals are too soft and malleable.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title properties of pure metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






