Properties of metals on the periodic table
Properties Of Metals On The Periodic Table. The line begins at boron b and extends down to polonium po. Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. Since its inception the periodic table has evolved time and again due to the discoveries of certain new metals and their properties. They are malleable they can be easily hammered into very thin sheets.
 The Periodic Table Metals Nonmetals And Metalloids Dummies From dummies.com
The Periodic Table Metals Nonmetals And Metalloids Dummies From dummies.com
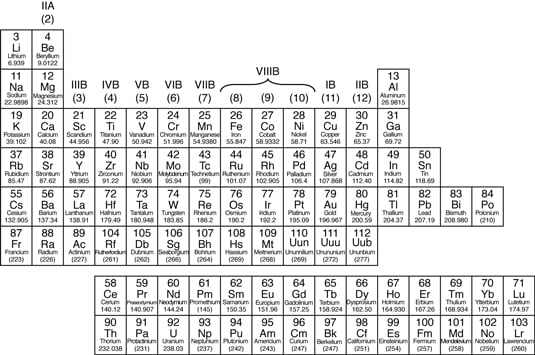
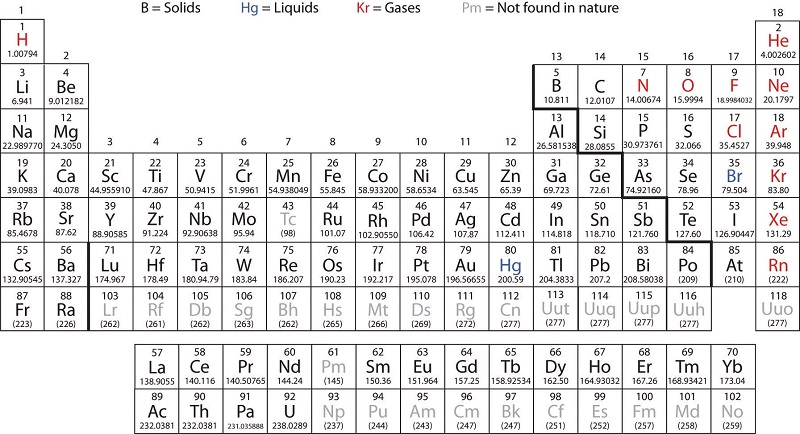
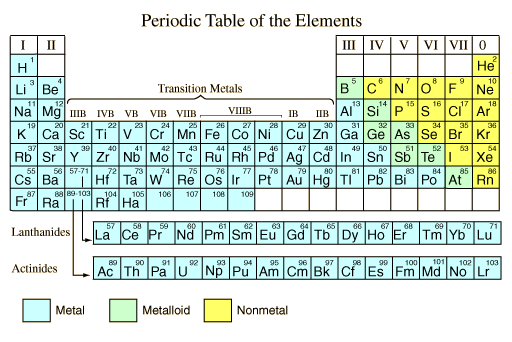
Properties of metals metalloids and nonmetals. Since its inception the periodic table has evolved time and again due to the discoveries of certain new metals and their properties. The metals in the periodic table. The following figure shows the metals. Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. All these metals tend to lose electrons easily.
They are ductile they can be drawn into thin wires.
Properties of metals metals shiny solids are room temperature except mercury which is a shiny liquid element with characteristic high melting points and densities. The arrangement of metals is organized with a view to making their identification. Are good conductors of heat and electricity. On most versions of the periodic table hydrogen is placed with the metals even though it has physical properties similar to those of the non metals it is a gas at room temperature. Properties of metals metalloids and nonmetals. The metalloids separate the metals and nonmetals on a periodic table.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Most metals have high melting and boiling points most non metals have low melting and boiling points. They are ductile they can be drawn into thin wires. Also many periodic tables have a stair step line on the table identifying the element groups. Properties of metals metalloids and nonmetals. All metals have a shiny appearance at least when freshly polished.
 Source: jagranjosh.com
Source: jagranjosh.com
Hydrogen is placed with the metals because it tends to behave like the other members of its column in chemical reactions. Metal and non metal elements have different physical properties. All metals have a shiny appearance at least when freshly polished. The arrangement of metals is organized with a view to making their identification. Good conductor of heat.
 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
The line begins at boron b and extends down to polonium po. Also many periodic tables have a stair step line on the table identifying the element groups. Good conductor of electricity. That s why elements show periodicity in their physical and chemical properties in the periodic table. The metals in the periodic table.
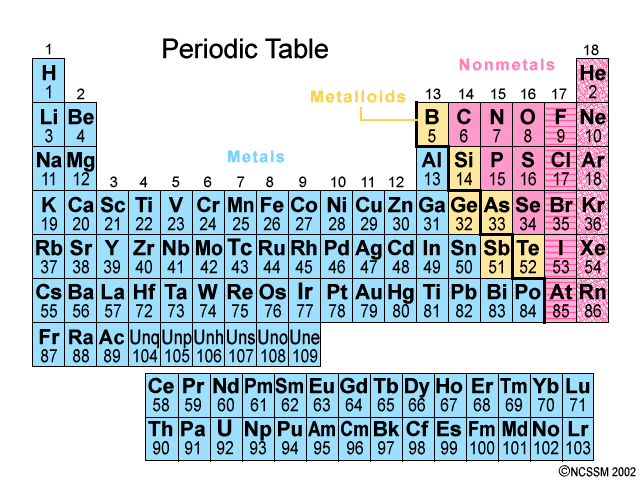 Source: socratic.org
Source: socratic.org
Are good conductors of heat and electricity. The following figure shows the metals. On most versions of the periodic table hydrogen is placed with the metals even though it has physical properties similar to those of the non metals it is a gas at room temperature. The bottom two rows of elements beneath the body of the periodic table are the lanthanides and actinides which are also metals. Periods 1 7 blocks s p d f the chemical elements can be broadly divided into metals metalloids and nonmetals according to their shared physical and chemical properties.
 Source: sciencenotes.org
Source: sciencenotes.org
The arrangement of metals is organized with a view to making their identification. The metals share several common properties including. Most metals have high melting and boiling points most non metals have low melting and boiling points. The bottom two rows of elements beneath the body of the periodic table are the lanthanides and actinides which are also metals. That s why elements show periodicity in their physical and chemical properties in the periodic table.
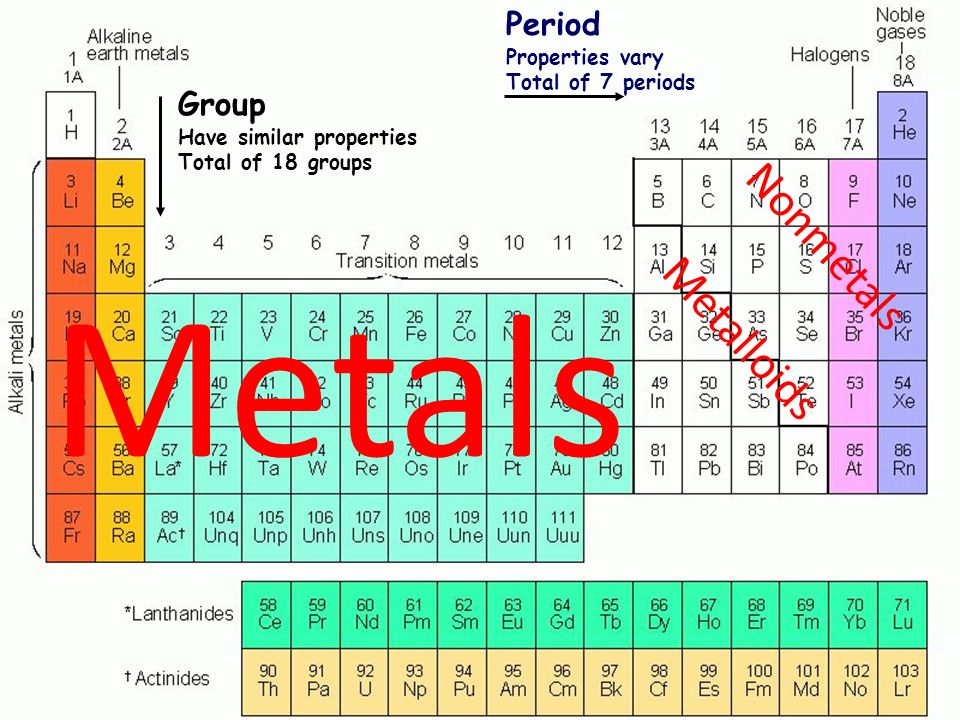 Source: slideplayer.com
Source: slideplayer.com
The line begins at boron b and extends down to polonium po. They are ductile they can be drawn into thin wires. Good conductor of electricity. Periods 1 7 blocks s p d f the chemical elements can be broadly divided into metals metalloids and nonmetals according to their shared physical and chemical properties. In modern periodic table elements have been arranged according to their atomic numbers and as stated above atomic numbers are directly related to their physical and chemical properties.
 Source: dummies.com
Source: dummies.com
Are good conductors of heat and electricity. Elements to the left of the line are considered metals. Solid at room temperature with the exception of mercury usually shiny. Properties of metals metalloids and nonmetals. Hydrogen is placed with the metals because it tends to behave like the other members of its column in chemical reactions.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The following figure shows the metals. They are malleable they can be easily hammered into very thin sheets. That s why elements show periodicity in their physical and chemical properties in the periodic table. The metalloids separate the metals and nonmetals on a periodic table. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals.
 Source: chemicool.com
Source: chemicool.com
Periods 1 7 blocks s p d f the chemical elements can be broadly divided into metals metalloids and nonmetals according to their shared physical and chemical properties. Good conductor of heat. Most metals have high melting and boiling points most non metals have low melting and boiling points. Solid at room temperature with the exception of mercury usually shiny. All these metals tend to lose electrons easily.
 Source: schoolworkhelper.net
Source: schoolworkhelper.net
Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. Most metals have high melting and boiling points most non metals have low melting and boiling points. The following figure shows the metals. Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. The bottom two rows of elements beneath the body of the periodic table are the lanthanides and actinides which are also metals.
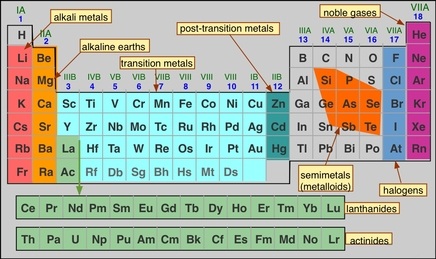 Source: online-sciences.com
Source: online-sciences.com
The metalloids separate the metals and nonmetals on a periodic table. Periods 1 7 blocks s p d f the chemical elements can be broadly divided into metals metalloids and nonmetals according to their shared physical and chemical properties. All these metals tend to lose electrons easily. That s why elements show periodicity in their physical and chemical properties in the periodic table. Also many periodic tables have a stair step line on the table identifying the element groups.
 Source: dummies.com
Source: dummies.com
Periods 1 7 blocks s p d f the chemical elements can be broadly divided into metals metalloids and nonmetals according to their shared physical and chemical properties. The arrangement of metals is organized with a view to making their identification. Good conductor of heat. Properties of metals metalloids and nonmetals. Since its inception the periodic table has evolved time and again due to the discoveries of certain new metals and their properties.
 Source: employees.csbsju.edu
Source: employees.csbsju.edu
The bottom two rows of elements beneath the body of the periodic table are the lanthanides and actinides which are also metals. The line begins at boron b and extends down to polonium po. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. The arrangement of metals is organized with a view to making their identification.
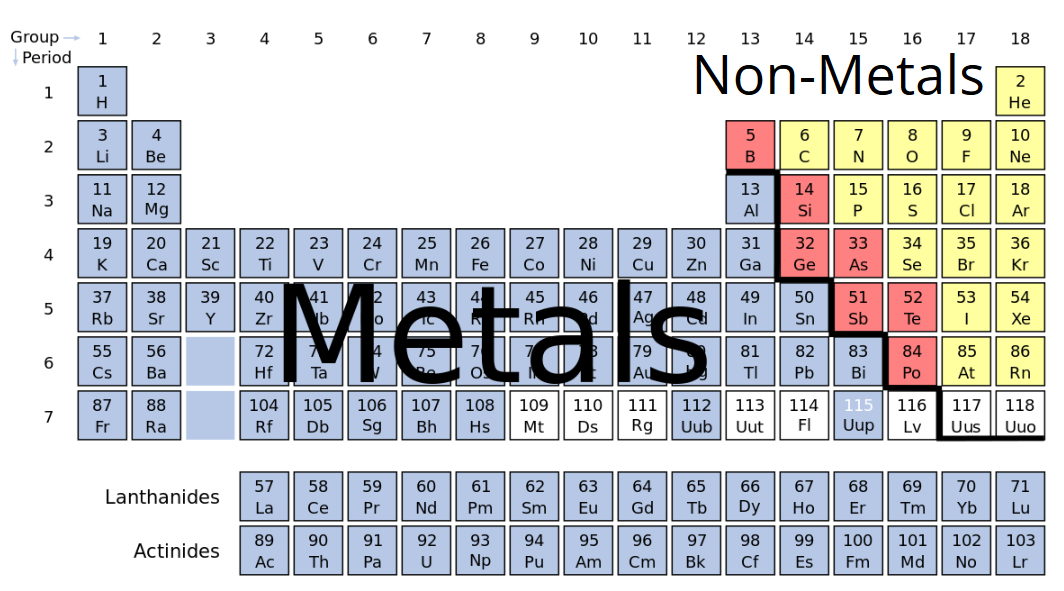 Source: sciencetrends.com
Source: sciencetrends.com
All these metals tend to lose electrons easily. In modern periodic table elements have been arranged according to their atomic numbers and as stated above atomic numbers are directly related to their physical and chemical properties. Most metals have high melting and boiling points most non metals have low melting and boiling points. The following figure shows the metals. All these metals tend to lose electrons easily.
 Source: wikihow.com
Source: wikihow.com
Periods 1 7 blocks s p d f the chemical elements can be broadly divided into metals metalloids and nonmetals according to their shared physical and chemical properties. Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. All metals have a shiny appearance at least when freshly polished. Metals in the periodic table so because most elements of the table are metals it makes sense to begin by looking at them. The bottom two rows of elements beneath the body of the periodic table are the lanthanides and actinides which are also metals.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title properties of metals on the periodic table by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.