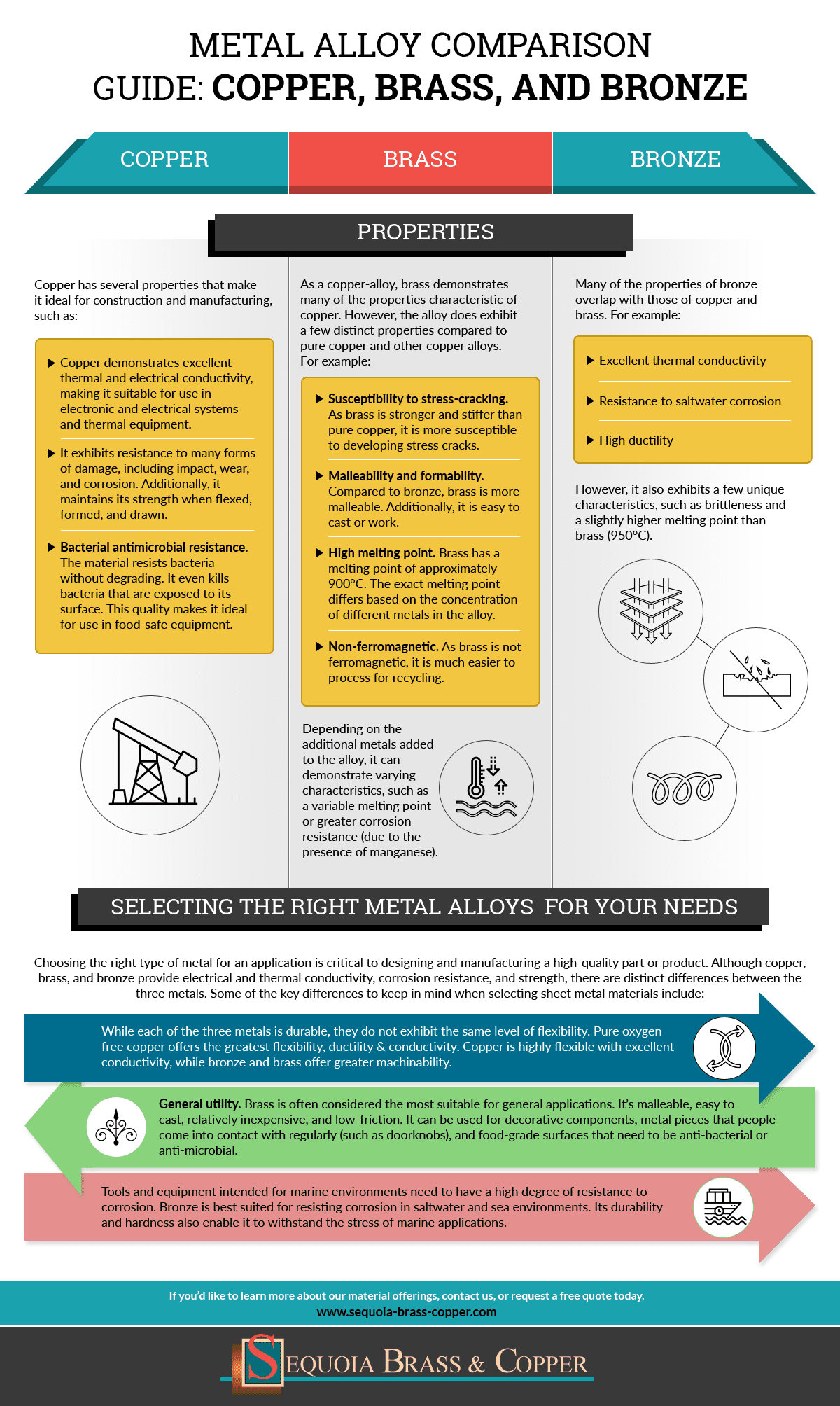
Properties of alloys compared to pure metals
Properties Of Alloys Compared To Pure Metals. The advantage of alloys differs depending on the specific alloy. List three properties you would expect a pure metal object to have. Metals are very important substances that we use in our day to day life. However the properties which alloys exhibit are different than the individual properties of these elements.
 Difference Between Silver And Stainless Steel Definition Properties Forms Types From pediaa.com
Difference Between Silver And Stainless Steel Definition Properties Forms Types From pediaa.com
The properties exhibited by alloys are often quite different from the properties of its individual components. Pure metals are too soft and malleable. Most pure metals are not used in manufacturing due to their softness when compared to alloys. Another important alloy of gold is white gold. An alloy is defined as a mixture of a metal with at least one other element. Bronze is made up of the pure metal copper.
Metals are very important substances that we use in our day to day life.
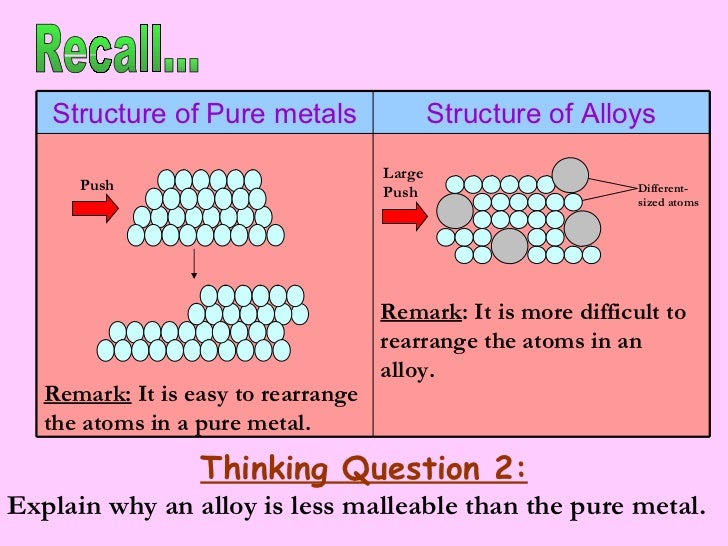
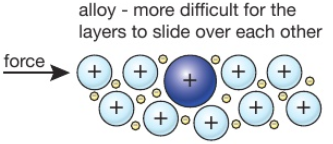
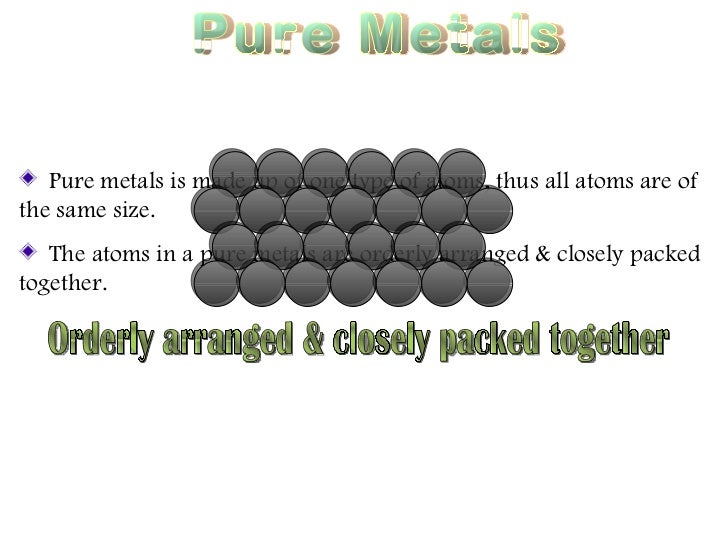
An example of an alloy is red gold which is produced by alloying copper and gold together. Although the forces of attraction between the atoms are strong pure metals are weak and soft due to their ductility and malleability and have limited use. Jewelry manufacturers alloy pure gold with silver copper or zinc to improve the metal s durability and rigidity. The key difference between metal and alloy is that the metal is a pure. Pure metals have a more uniform layer of atoms which also makes the atom layers more vulnerable to movement. Metals are very important substances that we use in our day to day life.
 Source: sequoia-brass-copper.com
Source: sequoia-brass-copper.com
Commercial metal alloys attempt to combine these beneficial properties in order to create metals more useful for particular applications than any of their component elements. Although the forces of attraction between the atoms are strong pure metals are weak and soft due to their ductility and malleability and have limited use. Pure metals may react with air and water i e. Pure metals are malleable weak and can be shaped. Most alloys are formed for one or two specific properties like strength and rust resistance.
 Source: pediaa.com
Source: pediaa.com
Alloys are used much more than pure metals because they are generally stronger and less likely to react with air or water. It is not uncommon for alloys to have greater strength and hardness when compared to pure metals. The properties exhibited by alloys are often quite different from the properties of its individual components. Some are better than pure materials while some are worse. An example of an alloy is red gold which is produced by alloying copper and gold together.
 Source: differencebetween.com
Source: differencebetween.com
Alloy is a subcategory of metal. When you compare it to the pure metals alloys are stronger and harder. An example of an alloy is red gold which is produced by alloying copper and gold together. Alloys are used much more than pure metals because they are generally stronger and less likely to react with air or water. Summary metal vs alloy.
 Source: youtube.com
Source: youtube.com
Some alloys also enhances the properties of the pure metal increasing its usefulness. Some alloys also enhances the properties of the pure metal increasing its usefulness. The properties exhibited by alloys are often quite different from the properties of its individual components. Pure metals are too soft and malleable. Summary metal vs alloy.
 Source: slideshare.net
Source: slideshare.net
They can also be formed when metals are combined with other elements. However the properties which alloys exhibit are different than the individual properties of these elements. When you compare it to the pure metals alloys are stronger and harder. Alloys are used much more than pure metals because they are generally stronger and less likely to react with air or water. An alloy is defined as a mixture of a metal with at least one other element.
 Source: slideshare.net
Source: slideshare.net
Although the forces of attraction between the atoms are strong pure metals are weak and soft due to their ductility and malleability and have limited use. Pure metals may be too soft to hold up to regular use but alloying them makes them tougher. Some alloys also enhances the properties of the pure metal increasing its usefulness. Pure metals have many useful properties but they are not widely used because of two main reasons. Alloy is a subcategory of metal.
 Source: slideshare.net
Source: slideshare.net
Summary metal vs alloy. Pure metals are ductile and soft because all the atoms are of the same size and orderly arranged thus the layers of atoms can slide over one another easily when a force is applied. Pure metals have a more uniform layer of atoms which also makes the atom layers more vulnerable to movement. Pure metals may react with air and water i e. However the properties which alloys exhibit are different than the individual properties of these elements.
 Source: slideplayer.com
Source: slideplayer.com
An alloy is a substance which is formed when two or more metals are combined. Pure metals have a more uniform layer of atoms which also makes the atom layers more vulnerable to movement. List three properties you would expect a pure metal object to have. Alloys are used much more than pure metals because they are generally stronger and less likely to react with air or water. Some are better than pure materials while some are worse.
 Source: tutormyself.com
Source: tutormyself.com
List three properties you would expect a pure metal object to have. Alloys are used much more than pure metals because they are generally stronger and less likely to react with air or water. Pure metals may react with air and water i e. As such we often use alloys instead of the pure metals. Individual pure metals may possess useful properties such as good electrical conductivity high strength and hardness or heat and corrosion resistance.
 Source: slideshare.net
Source: slideshare.net
Compare and contrast the general properties of alloys and pure metals. Compared to pure metals alloys can be stronger more resistant to damage and more versatile. Individual pure metals may possess useful properties such as good electrical conductivity high strength and hardness or heat and corrosion resistance. Most alloys are formed for one or two specific properties like strength and rust resistance. Another important alloy of gold is white gold.
 Source: differencebetween.com
Source: differencebetween.com
Compare and contrast the general properties of alloys and pure metals. List three properties you would expect a pure metal object to have. Some alloys also enhances the properties of the pure metal increasing its usefulness. An example of an alloy is red gold which is produced by alloying copper and gold together. Jewelry manufacturers alloy pure gold with silver copper or zinc to improve the metal s durability and rigidity.
 Source: simplechemconcepts.com
Source: simplechemconcepts.com
Bronze is made up of the pure metal copper. It is not uncommon for alloys to have greater strength and hardness when compared to pure metals. Pure metals may be too soft to hold up to regular use but alloying them makes them tougher. Most alloys are formed for one or two specific properties like strength and rust resistance. Alloy is a subcategory of metal.
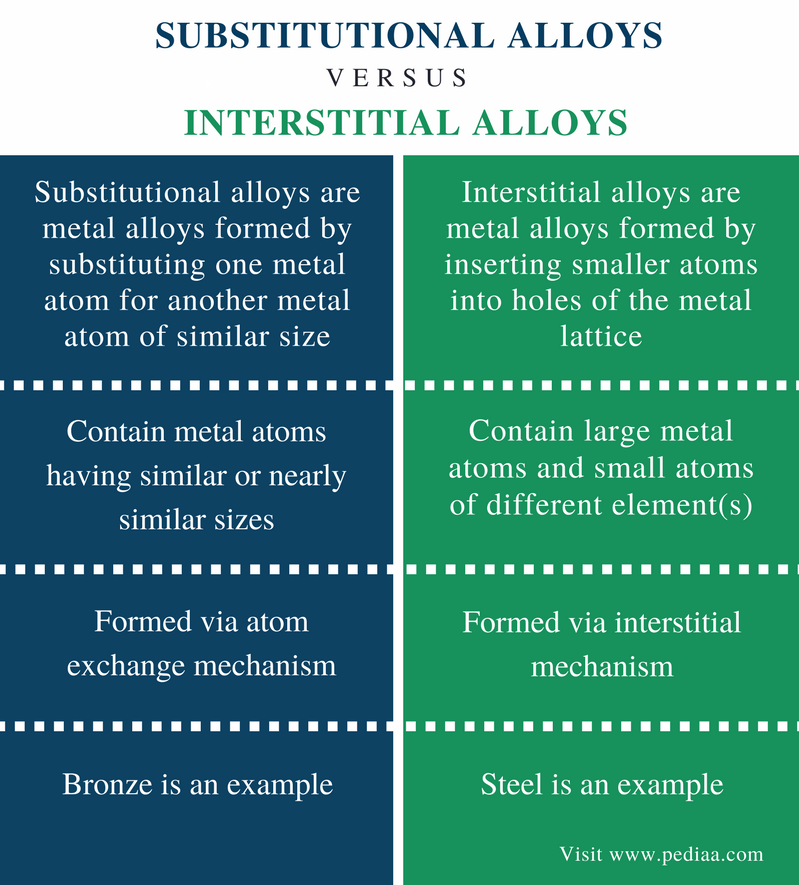 Source: pediaa.com
Source: pediaa.com
Although the forces of attraction between the atoms are strong pure metals are weak and soft due to their ductility and malleability and have limited use. An alloy is defined as a mixture of a metal with at least one other element. Bronze is made up of the pure metal copper. An example of an alloy is red gold which is produced by alloying copper and gold together. Pure metals are ductile and soft because all the atoms are of the same size and orderly arranged thus the layers of atoms can slide over one another easily when a force is applied.
 Source: slideshare.net
Source: slideshare.net
The key difference between metal and alloy is that the metal is a pure. They can also be formed when metals are combined with other elements. Pure metals have many useful properties but they are not widely used because of two main reasons. Some alloys also enhances the properties of the pure metal increasing its usefulness. Some are better than pure materials while some are worse.

The key difference between metal and alloy is that the metal is a pure. One reason that manufacturers combine pure metals to form alloys is to change the physical properties of the metals. An example of an alloy is red gold which is produced by alloying copper and gold together. When you compare it to the pure metals alloys are stronger and harder. Pure metals may react with air and water i e.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title properties of alloys compared to pure metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





