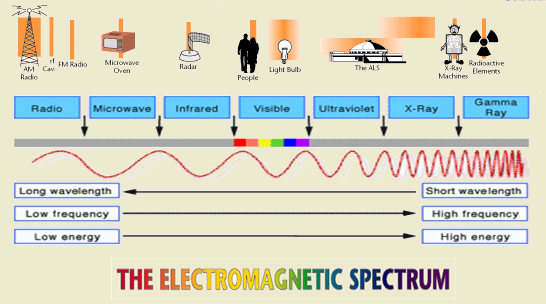
Periodic table characteristics
Periodic Table Characteristics. The elements are arranged in order of increasing atomic numbers in horizontal rows called periods and vertical columns called groups. All these metals tend to lose electrons easily. These trends can be predicted merely by examing the periodic table and can be explained and understood by analyzing the electron configurations of the elements. Radius calculated empirical covalent van der waals.
 Periodic Table Wikipedia From en.wikipedia.org
Periodic Table Wikipedia From en.wikipedia.org
One reason the periodic table of the elements is so useful is that it is a means of arranging elements according to their similar properties. The metals in the periodic table. They are shiny good conductors of electricity and heat. They are malleable they can be easily hammered into very thin sheets. Radius calculated empirical covalent van der waals. Characteristics of groups and periods in a periodic table.
Radius calculated empirical covalent van der waals.
The elements are arranged in order of increasing atomic numbers in horizontal rows called periods and vertical columns called groups. Elements tend to gain or lose valence electrons to achieve stable octet formation. The metals in the periodic table. They are malleable they can be easily hammered into very thin sheets. This is what is meant by periodicity or periodic table trends. Ionization 1st 2nd 3rd 4th 5th 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th 18th 19th 20th 21st 22nd 23rd 24th 25th 26th 27th 28th 29th 30th.
 Source: quora.com
Source: quora.com
Across a group as we move top to bottom in a group of the periodic table the metallic character of elements increases. This is what is meant by periodicity or periodic table trends. Characteristics of groups and periods in a periodic table. The metals in the periodic table. They are ductile they can be drawn into thin wires.
 Source: kullabs.com
Source: kullabs.com
The elements of a group show similar chemical properties but there is a gradual variation in the physical properties of the elements in a group. Metals reside on the left side of the table while non metals reside on the right. From top to bottom in a group the size of atom increases as a new shell to the atom. The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties. Characteristics of groups and periods in a periodic table.
 Source: pinterest.com
Source: pinterest.com
Why arrange elements in a table. Non metallic character of the elements across a period as we move left to right across a period in the periodic table nonmetallic character of elements increases. The elements of a group show similar chemical properties but there is a gradual variation in the physical properties of the elements in a group. Modern periodic table is based on the modern periodic law which states that the physical and chemical properties of the elements are the periodic function of their atomic numbers. They are ductile they can be drawn into thin wires.
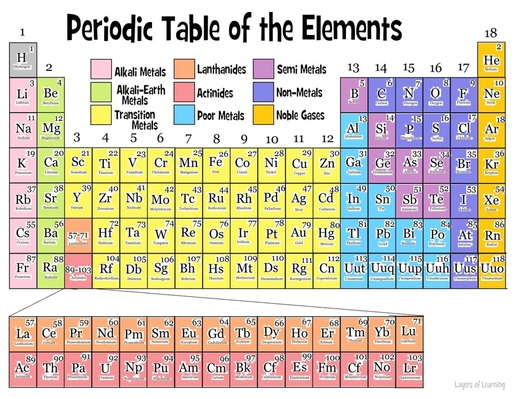 Source: periodic-table-components.weebly.com
Source: periodic-table-components.weebly.com
There are multiple ways of grouping the elements but they are commonly divided into metals semimetals metalloids and nonmetals. From top to bottom in a group the size of atom increases as a new shell to the atom. Ionization 1st 2nd 3rd 4th 5th 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th 18th 19th 20th 21st 22nd 23rd 24th 25th 26th 27th 28th 29th 30th. The creator of the periodic table dmitri mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. They are malleable they can be easily hammered into very thin sheets.
 Source: toppr.com
Source: toppr.com
There are multiple ways of grouping the elements but they are commonly divided into metals semimetals metalloids and nonmetals. The metals in the periodic table. They are shiny good conductors of electricity and heat. Elements tend to gain or lose valence electrons to achieve stable octet formation. He noticed that there were groups of elements that exhibited similar properties but he also noticed that there were plenty of exceptions to the.
 Source: youtube.com
Source: youtube.com
These trends can be predicted merely by examing the periodic table and can be explained and understood by analyzing the electron configurations of the elements. They are shiny good conductors of electricity and heat. Why arrange elements in a table. These trends can be predicted merely by examing the periodic table and can be explained and understood by analyzing the electron configurations of the elements. Elements tend to gain or lose valence electrons to achieve stable octet formation.
 Source: slideplayer.com
Source: slideplayer.com
They are ductile they can be drawn into thin wires. Across a group as we move top to bottom in a group of the periodic table the metallic character of elements increases. Ionization 1st 2nd 3rd 4th 5th 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th 18th 19th 20th 21st 22nd 23rd 24th 25th 26th 27th 28th 29th 30th. Elements tend to gain or lose valence electrons to achieve stable octet formation. They are malleable they can be easily hammered into very thin sheets.
 Source: slideshare.net
Source: slideshare.net
They are shiny good conductors of electricity and heat. He noticed that there were groups of elements that exhibited similar properties but he also noticed that there were plenty of exceptions to the. The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties. One reason the periodic table of the elements is so useful is that it is a means of arranging elements according to their similar properties. Characteristics of groups and periods in a periodic table.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The following figure shows the metals. The metals in the periodic table. Non metallic character of the elements across a period as we move left to right across a period in the periodic table nonmetallic character of elements increases. Modern periodic table is based on the modern periodic law which states that the physical and chemical properties of the elements are the periodic function of their atomic numbers. They are shiny good conductors of electricity and heat.
 Source: britannica.com
Source: britannica.com
Non metallic character of the elements across a period as we move left to right across a period in the periodic table nonmetallic character of elements increases. They are malleable they can be easily hammered into very thin sheets. Modern periodic table is based on the modern periodic law which states that the physical and chemical properties of the elements are the periodic function of their atomic numbers. Elements tend to gain or lose valence electrons to achieve stable octet formation. He noticed that there were groups of elements that exhibited similar properties but he also noticed that there were plenty of exceptions to the.
 Source: brainly.in
Source: brainly.in
The creator of the periodic table dmitri mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train. Why arrange elements in a table. Across a group as we move top to bottom in a group of the periodic table the metallic character of elements increases. This is what is meant by periodicity or periodic table trends. The following figure shows the metals.
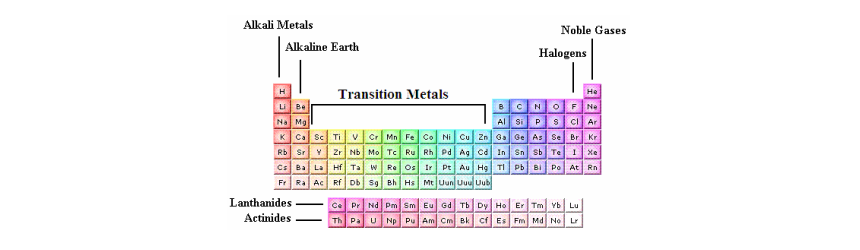 Source: 170188733453308075.weebly.com
Source: 170188733453308075.weebly.com
The elements of a group show similar chemical properties but there is a gradual variation in the physical properties of the elements in a group. The periodic table arranges the elements by periodic properties which are recurring trends in physical and chemical characteristics. Radius calculated empirical covalent van der waals. They are malleable they can be easily hammered into very thin sheets. Ionization 1st 2nd 3rd 4th 5th 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th 18th 19th 20th 21st 22nd 23rd 24th 25th 26th 27th 28th 29th 30th.
 Source: kullabs.com
Source: kullabs.com
Modern periodic table is based on the modern periodic law which states that the physical and chemical properties of the elements are the periodic function of their atomic numbers. Elements tend to gain or lose valence electrons to achieve stable octet formation. The elements are arranged in order of increasing atomic numbers in horizontal rows called periods and vertical columns called groups. Characteristics of groups and periods in a periodic table. The elements of a group show similar chemical properties but there is a gradual variation in the physical properties of the elements in a group.
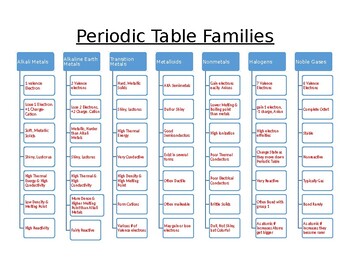 Source: teacherspayteachers.com
Source: teacherspayteachers.com
They are shiny good conductors of electricity and heat. They are ductile they can be drawn into thin wires. Across a group as we move top to bottom in a group of the periodic table the metallic character of elements increases. Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world but it was not always so obvious. The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties.
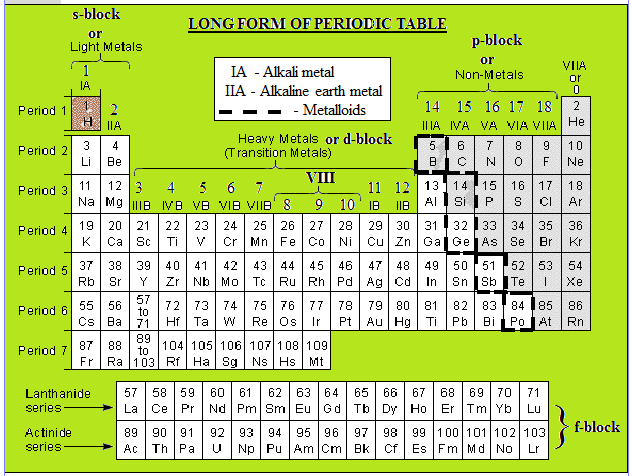 Source: freakgenie.com
Source: freakgenie.com
Elements tend to gain or lose valence electrons to achieve stable octet formation. They are ductile they can be drawn into thin wires. Elements tend to gain or lose valence electrons to achieve stable octet formation. These trends can be predicted merely by examing the periodic table and can be explained and understood by analyzing the electron configurations of the elements. The elements are arranged in order of increasing atomic numbers in horizontal rows called periods and vertical columns called groups.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title periodic table characteristics by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.