Oxygen metal or nonmetal
Oxygen Metal Or Nonmetal. 1 when sulphur burns in air it combines with the oxygen of air to form sulphur dioxide acidic oxide s s o2 g so2 g sulphur dioxide dissolves in water to form sulphurous acid solution. The physical properties are insufficient to identify if a substance is metal or a nonmetal and so there is need to test chemical properties of metal and non metal. Metal react with oxygen to form metal oxide metal oxygen metal oxide. Lets start with reaction of metals with oxygen.
 Reaction Of Non Metals With Oxygen Youtube From youtube.com
Reaction Of Non Metals With Oxygen Youtube From youtube.com
Reaction with oxygen metals. The physical properties are insufficient to identify if a substance is metal or a nonmetal and so there is need to test chemical properties of metal and non metal. Non metals are not able to conduct electricity or heat very well. As opposed to metals non metallic elements are very brittle and cannot be rolled into wires or pounded into sheets. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. Metal react with oxygen to form metal oxide metal oxygen metal oxide.
Lets start with reaction of metals with oxygen.
It belongs to group 16 or chalcogens. Lets start with reaction of metals with oxygen. Sodium reacts vigorously with the oxygen present in air. Reaction with oxygen reaction with water reaction with acids reaction with bases and displacement reactions. Most metals combine with oxygen to form metal oxides. 1 when sulphur burns in air it combines with the oxygen of air to form sulphur dioxide acidic oxide s s o2 g so2 g sulphur dioxide dissolves in water to form sulphurous acid solution.
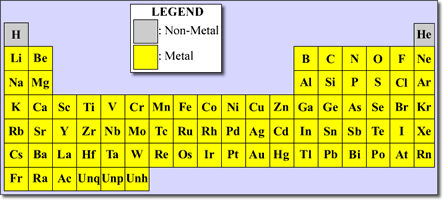 Source: astronomy.swin.edu.au
Source: astronomy.swin.edu.au
Non metals are not able to conduct electricity or heat very well. Chemical properties of metals and nonmetals chemical properties of metals and non metals can be divided into five categories. Metal react with oxygen to form metal oxide metal oxygen metal oxide. As opposed to metals non metallic elements are very brittle and cannot be rolled into wires or pounded into sheets. Most metals combine with oxygen to form metal oxides.
 Source: intl.siyavula.com
Source: intl.siyavula.com
1 when sulphur burns in air it combines with the oxygen of air to form sulphur dioxide acidic oxide s s o2 g so2 g sulphur dioxide dissolves in water to form sulphurous acid solution. The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. As opposed to metals non metallic elements are very brittle and cannot be rolled into wires or pounded into sheets. The physical properties are insufficient to identify if a substance is metal or a nonmetal and so there is need to test chemical properties of metal and non metal. Reaction with oxygen metals.
 Source: teachoo.com
Source: teachoo.com
Metal react with oxygen to form metal oxide metal oxygen metal oxide. Lets start with reaction of metals with oxygen. Most metals combine with oxygen to form metal oxides. It belongs to group 16 or chalcogens. Reaction with oxygen metals.
Source: quora.com
The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. Most metals combine with oxygen to form metal oxides. Oxygen is a non metal a gas. Non metals are not able to conduct electricity or heat very well. As opposed to metals non metallic elements are very brittle and cannot be rolled into wires or pounded into sheets.

Reaction with oxygen metals. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. Elements on the left hand side with the e. Sodium reacts vigorously with the oxygen present in air. Oxygen is a non metal a gas.
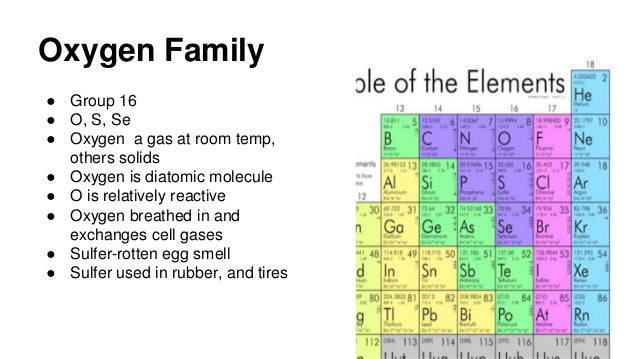 Source: slideshare.net
Source: slideshare.net
It belongs to group 16 or chalcogens. Oxygen is a non metal a gas. Lets start with reaction of metals with oxygen. It belongs to group 16 or chalcogens. To determine whether oxygen o is a metal nonmetal or metalloid we look at its position on the periodic table.
 Source: slideplayer.com
Source: slideplayer.com
As opposed to metals non metallic elements are very brittle and cannot be rolled into wires or pounded into sheets. Non metals are not able to conduct electricity or heat very well. As opposed to metals non metallic elements are very brittle and cannot be rolled into wires or pounded into sheets. Oxygen is not a metal nor a metalloid. To determine whether oxygen o is a metal nonmetal or metalloid we look at its position on the periodic table.
 Source: teachoo.com
Source: teachoo.com
Lets start with reaction of metals with oxygen. Non metals are not able to conduct electricity or heat very well. Lets start with reaction of metals with oxygen. Elements on the left hand side with the e. Sodium reacts vigorously with the oxygen present in air.
 Source: quora.com
Source: quora.com
It belongs to group 16 or chalcogens. 1 when sulphur burns in air it combines with the oxygen of air to form sulphur dioxide acidic oxide s s o2 g so2 g sulphur dioxide dissolves in water to form sulphurous acid solution. To determine whether oxygen o is a metal nonmetal or metalloid we look at its position on the periodic table. Sodium reacts vigorously with the oxygen present in air. Oxygen is a non metal a gas.
Source: quora.com
Lets start with reaction of metals with oxygen. Non metals react with oxygen to form non metal oxides non metal oxides are acidic in nature they turn blue litmus to red. Sodium reacts vigorously with the oxygen present in air. Most metals combine with oxygen to form metal oxides. The physical properties are insufficient to identify if a substance is metal or a nonmetal and so there is need to test chemical properties of metal and non metal.
 Source: quora.com
Source: quora.com
Oxygen is not a metal nor a metalloid. Metal react with oxygen to form metal oxide metal oxygen metal oxide. Chemical properties of metals and nonmetals chemical properties of metals and non metals can be divided into five categories. Reaction with oxygen metals. The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc.
 Source: toppr.com
Source: toppr.com
Reaction with oxygen metals. Lets start with reaction of metals with oxygen. Reaction with oxygen reaction with water reaction with acids reaction with bases and displacement reactions. 1 when sulphur burns in air it combines with the oxygen of air to form sulphur dioxide acidic oxide s s o2 g so2 g sulphur dioxide dissolves in water to form sulphurous acid solution. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc.
 Source: sciencenotes.org
Source: sciencenotes.org
Reaction with oxygen metals. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. Non metals are not able to conduct electricity or heat very well. 1 when sulphur burns in air it combines with the oxygen of air to form sulphur dioxide acidic oxide s s o2 g so2 g sulphur dioxide dissolves in water to form sulphurous acid solution. Most metals combine with oxygen to form metal oxides.
 Source: jagranjosh.com
Source: jagranjosh.com
Metal react with oxygen to form metal oxide metal oxygen metal oxide. Reaction with oxygen metals. Oxygen is a non metal a gas. Sodium reacts vigorously with the oxygen present in air. Elements on the left hand side with the e.
 Source: youtube.com
Source: youtube.com
The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. Non metals react with oxygen to form non metal oxides non metal oxides are acidic in nature they turn blue litmus to red. Oxygen is not a metal nor a metalloid. Oxygen is a non metal a gas. Elements on the left hand side with the e.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title oxygen metal or nonmetal by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.