Non metals that conduct electricity
Non Metals That Conduct Electricity. Electrical conductivity in metals is due to the presence of free electrons which are absent in non metals by free free to move electrons we mean the electrons that are loosely bound to the nuclei. Silicon and germanium are used commercially for their ability to conduct electricity. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. Non metals are usually poor conductors of heat and electricity.
 Can Non Metal Conduct Electricity If Yes How If No Why Socratic From socratic.org
Can Non Metal Conduct Electricity If Yes How If No Why Socratic From socratic.org
Some non metals are very reactive whereas other are not reactive at all. Silicon and germanium are used commercially for their ability to conduct electricity. I hope it will help you. Other nonmetals are electrical insulators. Follow me and mark me as brilliant. Metalloids are a group of related nonmetal elements with some metal traits including the ability to conduct electricity.
It doesn t look metallic can t be made into a wire pounded into shape or bent doesn t conduct heat or electricity well and doesn t have a high melting or boiling point.
Non metals are usually poor conductors of heat and electricity. Br a name a lustrous non metal. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. It depends on the number of electrons in there outer energy level reactive non metals tend to gain electrons. A nonmetal is simply an element that does not display the properties of a metal it is not defined by what it is but by what it is not. Most of the non metals do not conduct electricity but there are some exceptions like graphite silicon semi conductor and metalloids also semiconductors.
 Source: youtube.com
Source: youtube.com
The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. Some non metals are very reactive whereas other are not reactive at all. The metalloids are boron silicon germanium arsenic antimony and tellurium. Br c the allotropic form of a non metal is a good conductor of electricity. Metalloids are a group of related nonmetal elements with some metal traits including the ability to conduct electricity.

It depends on the number of electrons in there outer energy level reactive non metals tend to gain electrons. Br c the allotropic form of a non metal is a good conductor of electricity. I hope it will help you. A nonmetal is simply an element that does not display the properties of a metal it is not defined by what it is but by what it is not. It doesn t look metallic can t be made into a wire pounded into shape or bent doesn t conduct heat or electricity well and doesn t have a high melting or boiling point.
 Source: ssc.education.ed.ac.uk
Source: ssc.education.ed.ac.uk
Non metals are usually poor conductors of heat and electricity. Non metals are natural materials that do not produce heat or electricity and that are structurally brittle can not be easily rolling moulding extruding or pressing. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. Silicon and germanium are used commercially for their ability to conduct electricity. Metalloids are a group of related nonmetal elements with some metal traits including the ability to conduct electricity.
 Source: slideplayer.com
Source: slideplayer.com
Silver is not always an ideal choice as a material however because it is expensive and susceptible to tarnishing and the oxide layer known as tarnish is not conductive. This explain why they cannot conduct electricity which is a flow of electrons. The metalloids are boron silicon germanium arsenic antimony and tellurium. Silicon and germanium are used commercially for their ability to conduct electricity. Non metals are natural materials that do not produce heat or electricity and that are structurally brittle can not be easily rolling moulding extruding or pressing.
 Source: pt.slideshare.net
Source: pt.slideshare.net
They are non lustrous non sonorous non malleable and are coloured. It depends on the number of electrons in there outer energy level reactive non metals tend to gain electrons. They are non lustrous non sonorous non malleable and are coloured. The metalloids are boron silicon germanium arsenic antimony and tellurium. Silicon and germanium are used commercially for their ability to conduct electricity.
 Source: ssc.education.ed.ac.uk
Source: ssc.education.ed.ac.uk
The metalloids are boron silicon germanium arsenic antimony and tellurium. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. Silver is not always an ideal choice as a material however because it is expensive and susceptible to tarnishing and the oxide layer known as tarnish is not conductive. Non metals are usually poor conductors of heat and electricity. Metalloids are a group of related nonmetal elements with some metal traits including the ability to conduct electricity.
 Source: thoughtco.com
Source: thoughtco.com
Other nonmetals are electrical insulators. Most of the non metals do not conduct electricity but there are some exceptions like graphite silicon semi conductor and metalloids also semiconductors. A nonmetal is simply an element that does not display the properties of a metal it is not defined by what it is but by what it is not. Br a name a lustrous non metal. Chemically hydrogen carbon nitrogen oxygen phosphorus arsenic and selenium are the non metallic elements in the periodic table.
Source: quora.com
It depends on the number of electrons in there outer energy level reactive non metals tend to gain electrons. Non metals are usually poor conductors of heat and electricity. Follow me and mark me as brilliant. Br a name a lustrous non metal. This explain why they cannot conduct electricity which is a flow of electrons.
 Source: steemit.com
Source: steemit.com
Electrical conductivity in metals is due to the presence of free electrons which are absent in non metals by free free to move electrons we mean the electrons that are loosely bound to the nuclei. Follow me and mark me as brilliant. They are non lustrous non sonorous non malleable and are coloured. It doesn t look metallic can t be made into a wire pounded into shape or bent doesn t conduct heat or electricity well and doesn t have a high melting or boiling point. Br b name a non metal which exists as a liquid at room temperature.
 Source: scientificamerican.com
Source: scientificamerican.com
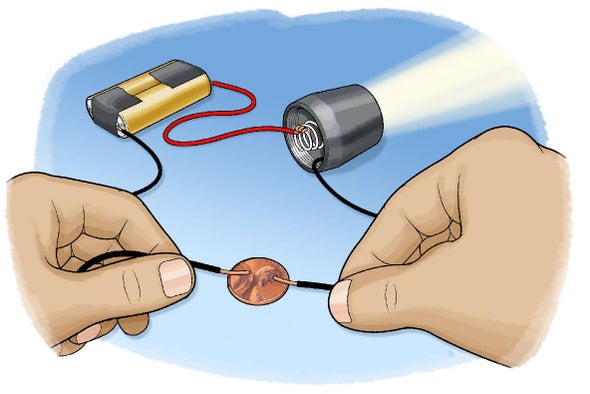
I hope it will help you. Br b name a non metal which exists as a liquid at room temperature. This explain why they cannot conduct electricity which is a flow of electrons. Chemically hydrogen carbon nitrogen oxygen phosphorus arsenic and selenium are the non metallic elements in the periodic table. Graphite is an allotrope of carbon and it has one electron per carbon atom that is delocalized and not involved in chemical bonding.
 Source: quora.com
Source: quora.com
Silver is not always an ideal choice as a material however because it is expensive and susceptible to tarnishing and the oxide layer known as tarnish is not conductive. Br a name a lustrous non metal. Electrical conductivity in metals is due to the presence of free electrons which are absent in non metals by free free to move electrons we mean the electrons that are loosely bound to the nuclei. It depends on the number of electrons in there outer energy level reactive non metals tend to gain electrons. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver.
 Source: slideplayer.com
Source: slideplayer.com
The metalloids are boron silicon germanium arsenic antimony and tellurium. Electrical conductivity in metals is due to the presence of free electrons which are absent in non metals by free free to move electrons we mean the electrons that are loosely bound to the nuclei. The metalloids are boron silicon germanium arsenic antimony and tellurium. Br b name a non metal which exists as a liquid at room temperature. It doesn t look metallic can t be made into a wire pounded into shape or bent doesn t conduct heat or electricity well and doesn t have a high melting or boiling point.
 Source: sciencenotes.org
Source: sciencenotes.org
The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. Br a name a lustrous non metal. Most of the non metals do not conduct electricity but there are some exceptions like graphite silicon semi conductor and metalloids also semiconductors. It depends on the number of electrons in there outer energy level reactive non metals tend to gain electrons. Br c the allotropic form of a non metal is a good conductor of electricity.
 Source: socratic.org
Source: socratic.org
Other nonmetals are electrical insulators. Most of the non metals do not conduct electricity but there are some exceptions like graphite silicon semi conductor and metalloids also semiconductors. Br a name a lustrous non metal. They are non lustrous non sonorous non malleable and are coloured. The metalloids are boron silicon germanium arsenic antimony and tellurium.
 Source: toppr.com
Source: toppr.com
Other nonmetals are electrical insulators. Silicon and germanium are used commercially for their ability to conduct electricity. Chemically hydrogen carbon nitrogen oxygen phosphorus arsenic and selenium are the non metallic elements in the periodic table. A nonmetal is simply an element that does not display the properties of a metal it is not defined by what it is but by what it is not. Silver is not always an ideal choice as a material however because it is expensive and susceptible to tarnishing and the oxide layer known as tarnish is not conductive.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title non metals that conduct electricity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.