Non metal characteristics
Non Metal Characteristics. Nonmetals display a wide range of chemical properties and reactivities. They are generally poor conductors of heat and electricity. Among the non metals the anionic dopants have a strong influence on the vb. Most nonmetals have the ability to gain electrons easily.
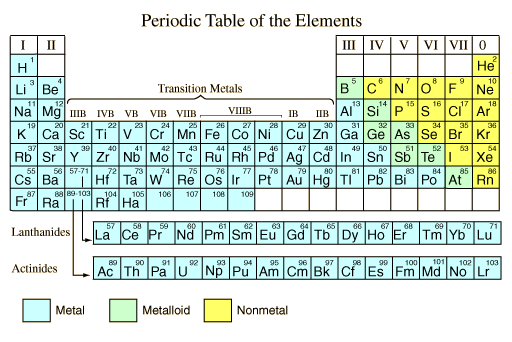 Metals And Nonmetals From hyperphysics.phy-astr.gsu.edu
Metals And Nonmetals From hyperphysics.phy-astr.gsu.edu
Usually their melting points are lower than for metals although there are exceptions. Nonmetals have high ionization energies and electronegativities. Properties of nonmetals. The solids usually break easily and can t bend like metals. In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Solid nonmetals are generally brittle with little or no metallic luster.
Solid nonmetals are generally brittle with little or no metallic luster.
Because of these properties non metals usually gain electrons when reacting with other compounds forming covalent bonds. Usually their melting points are lower than for metals although there are exceptions. Properties of nonmetals. Most nonmetals have the ability to gain electrons easily. In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Because of these properties non metals usually gain electrons when reacting with other compounds forming covalent bonds.
 Source: slideshare.net
Source: slideshare.net
Characteristics properties of non metals are high ionization energies and high electronegativity. They are generally poor conductors of heat and electricity. The solids usually break easily and can t bend like metals. Nonmetals display a wide range of chemical properties and reactivities. Solid nonmetals are generally brittle with little or no metallic luster.
 Source: researchgate.net
Source: researchgate.net
Nonmetals have high ionization energies and electronegativities. The solids usually break easily and can t bend like metals. Nonmetals display a wide range of chemical properties and reactivities. Solid nonmetals are generally brittle with little or no metallic luster. Most nonmetals have the ability to gain electrons easily.
 Source: slideplayer.com
Source: slideplayer.com
Nonmetals display a wide range of chemical properties and reactivities. In the elemental form non metals can be gas liquid or solid. Most nonmetals have the ability to gain electrons easily. Because of these properties non metals usually gain electrons when reacting with other compounds forming covalent bonds. Characteristics properties of non metals are high ionization energies and high electronegativity.
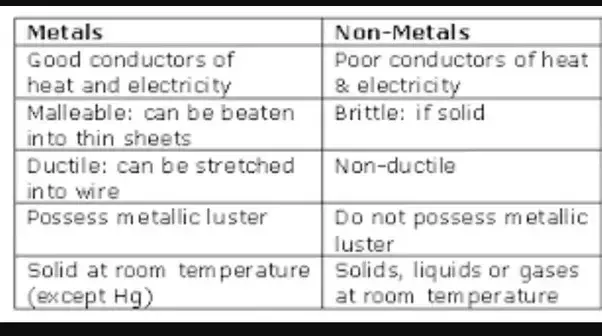 Source: quora.com
Source: quora.com
In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. In the elemental form non metals can be gas liquid or solid. Because of these properties non metals usually gain electrons when reacting with other compounds forming covalent bonds. Among the non metals the anionic dopants have a strong influence on the vb. Nonmetals have high ionization energies and electronegativities.
 Source: meritnation.com
Source: meritnation.com
Nonmetals have high ionization energies and electronegativities. They are generally poor conductors of heat and electricity. Usually their melting points are lower than for metals although there are exceptions. Non metal dopants are carbon nitrogen fluorine sulphur and iodine. Among the non metals the anionic dopants have a strong influence on the vb.
 Source: slideshare.net
Source: slideshare.net
In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Because of these properties non metals usually gain electrons when reacting with other compounds forming covalent bonds. In the elemental form non metals can be gas liquid or solid. Usually their melting points are lower than for metals although there are exceptions. Properties of nonmetals.
 Source: thoughtco.com
Source: thoughtco.com
They aren t shiny lustrous and they don t conduct heat or electricity well. In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Solid nonmetals are generally brittle with little or no metallic luster. Among the non metals the anionic dopants have a strong influence on the vb. Usually their melting points are lower than for metals although there are exceptions.
 Source: thoughtco.com
Source: thoughtco.com
In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Solid nonmetals are generally brittle with little or no metallic luster. In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Because of these properties non metals usually gain electrons when reacting with other compounds forming covalent bonds. The solids usually break easily and can t bend like metals.
 Source: pinterest.com
Source: pinterest.com
Among the non metals the anionic dopants have a strong influence on the vb. They aren t shiny lustrous and they don t conduct heat or electricity well. Solid nonmetals are generally brittle with little or no metallic luster. Non metal dopants are carbon nitrogen fluorine sulphur and iodine. Usually their melting points are lower than for metals although there are exceptions.

Because of these properties non metals usually gain electrons when reacting with other compounds forming covalent bonds. Nonmetals display a wide range of chemical properties and reactivities. Properties of nonmetals. Usually their melting points are lower than for metals although there are exceptions. The solids usually break easily and can t bend like metals.
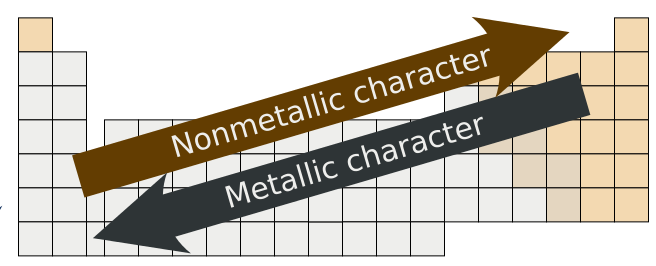 Source: chem.libretexts.org
Source: chem.libretexts.org
Properties of nonmetals. Usually their melting points are lower than for metals although there are exceptions. Because of these properties non metals usually gain electrons when reacting with other compounds forming covalent bonds. Nonmetals display a wide range of chemical properties and reactivities. In the elemental form non metals can be gas liquid or solid.

Most nonmetals have the ability to gain electrons easily. Because of these properties non metals usually gain electrons when reacting with other compounds forming covalent bonds. Nonmetals display a wide range of chemical properties and reactivities. Characteristics properties of non metals are high ionization energies and high electronegativity. Usually their melting points are lower than for metals although there are exceptions.
 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
Usually their melting points are lower than for metals although there are exceptions. Solid nonmetals are generally brittle with little or no metallic luster. Most nonmetals have the ability to gain electrons easily. In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. In the elemental form non metals can be gas liquid or solid.
 Source: slideshare.net
Source: slideshare.net
Among the non metals the anionic dopants have a strong influence on the vb. Characteristics properties of non metals are high ionization energies and high electronegativity. In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Non metal dopants are carbon nitrogen fluorine sulphur and iodine. They aren t shiny lustrous and they don t conduct heat or electricity well.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Characteristics properties of non metals are high ionization energies and high electronegativity. In chemistry a nonmetal or non metal is a chemical element that mostly lacks the characteristics of a metal physically a nonmetal tends to have a relatively low melting point boiling point and density a nonmetal is typically brittle when solid and usually has poor thermal conductivity and electrical conductivity chemically nonmetals tend to have relatively high ionization energy. Characteristics properties of non metals are high ionization energies and high electronegativity. Nonmetals have high ionization energies and electronegativities. In the elemental form non metals can be gas liquid or solid.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title non metal characteristics by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





