Name the properties of water
Name The Properties Of Water. Because of the polarity of the molecules water molecules are attracted to each other. Cohesion is a key property of water. Properties of water include its chemical formula h2o density melting boiling point how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. It helps organisms to tolerate pressure and compression.
 Properties Of Water Youtube From m.youtube.com
Properties Of Water Youtube From m.youtube.com
Cohesion is a key property of water. Water has a high polarity and it gives it the ability to being attracted to other water molecules. Water has high dielectric constant. It helps organisms to tolerate pressure and compression. Because of the polarity of the molecules water molecules are attracted to each other. Because of this in earthworm water acts as hydro skeleton.
There are several important properties of water that distinguish it from other molecules and make it the key compound for life.
Because of the polarity of the molecules water molecules are attracted to each other. It is one of its most important properties. Water has high dielectric constant. Cohesion is a key property of water. These molecules are held together by the hydrogen bonds in water. It helps organisms to tolerate pressure and compression.
 Source: owlcation.com
Source: owlcation.com
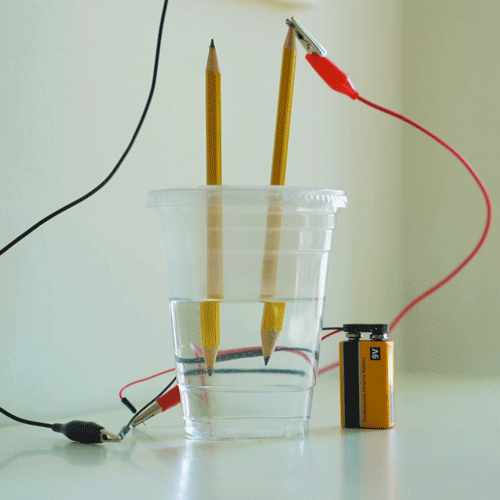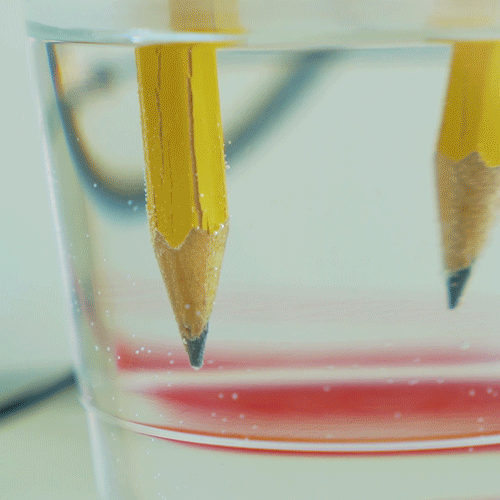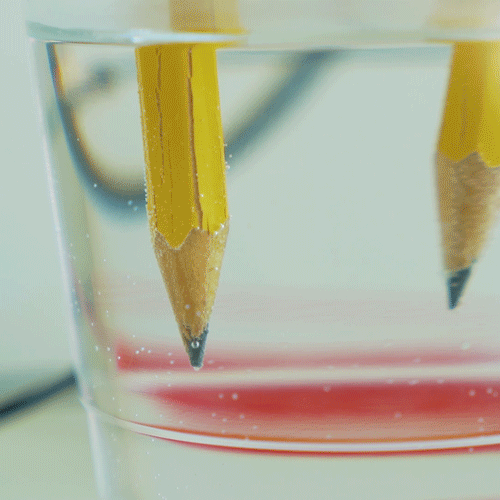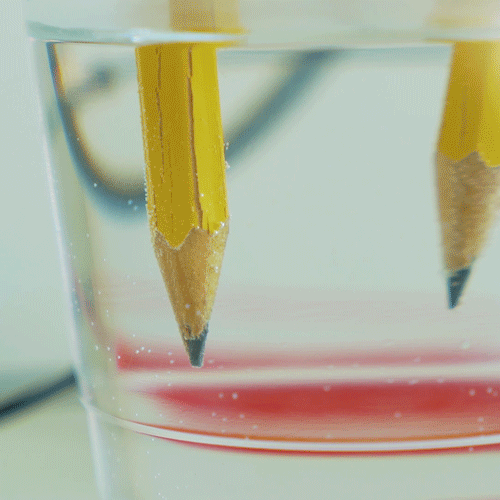
These two unusual properties allow water to moderate earth s. This four minute animation describes the properties of water that support life. Cohesion is what we call the ability of water to attract other water molecules. This opposes the attraction of opposite charges of ions. Cohesion is a key property of water.
 Source: slideplayer.com
Source: slideplayer.com
It helps organisms to tolerate pressure and compression. Water has a high polarity and it gives it the ability to being attracted to other water molecules. These properties include solvency cohesion and adhesion high surface temper. Water s high surface tension is due to the hydrogen bonding in water molecules. Cohesion is what we call the ability of water to attract other water molecules.
 Source: slideplayer.com
Source: slideplayer.com
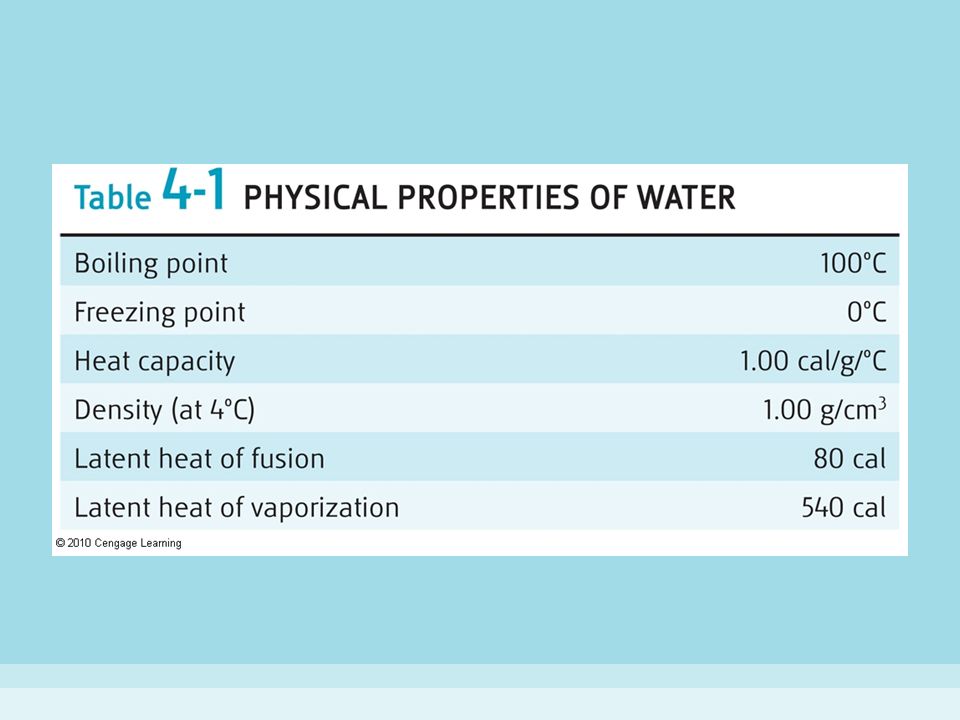
Water has a very high specific heat capacity of 4181 4 j kg k at 25 c the second highest among all the heteroatomic species after ammonia as well as a high heat of vaporization 40 65 kj mol or 2257 kj kg at the normal boiling point both of which are a result of the extensive hydrogen bonding between its molecules. It is one of its most important properties. Cohesion is a key property of water. There are several important properties of water that distinguish it from other molecules and make it the key compound for life. These molecules are held together by the hydrogen bonds in water.
 Source: slideshare.net
Source: slideshare.net
Cohesion high specific heat high heat of vaporization lower density of ice and high polarity. Water s high surface tension is due to the hydrogen bonding in water molecules. Water has a very high specific heat capacity of 4181 4 j kg k at 25 c the second highest among all the heteroatomic species after ammonia as well as a high heat of vaporization 40 65 kj mol or 2257 kj kg at the normal boiling point both of which are a result of the extensive hydrogen bonding between its molecules. Because of this water acts as powerful solvent for salts and many non ionizable organic molecules. Because of this in earthworm water acts as hydro skeleton.
 Source: slideshare.net
Source: slideshare.net
It is one of its most important properties. Water has the highest surface tension for all liquids. Water s high surface tension is due to the hydrogen bonding in water molecules. This opposes the attraction of opposite charges of ions. Cohesion is what we call the ability of water to attract other water molecules.
 Source: byjus.com
Source: byjus.com
These two unusual properties allow water to moderate earth s. Properties of water include its chemical formula h2o density melting boiling point how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. Because of this water acts as powerful solvent for salts and many non ionizable organic molecules. Because of the polarity of the molecules water molecules are attracted to each other. Cohesion is what we call the ability of water to attract other water molecules.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It helps organisms to tolerate pressure and compression. 5 properties of water. Learn about its physical chemical properties of water its importance for the existence of life. Water s high surface tension is due to the hydrogen bonding in water molecules. Cohesion high specific heat high heat of vaporization lower density of ice and high polarity.
 Source: slideserve.com
Source: slideserve.com
It is one of its most important properties. Cohesion high specific heat high heat of vaporization lower density of ice and high polarity. This opposes the attraction of opposite charges of ions. Water s high surface tension is due to the hydrogen bonding in water molecules. These molecules are held together by the hydrogen bonds in water.
Source:
This opposes the attraction of opposite charges of ions. Learn about its physical chemical properties of water its importance for the existence of life. Properties of water include its chemical formula h2o density melting boiling point how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. These two unusual properties allow water to moderate earth s. Because of this water acts as powerful solvent for salts and many non ionizable organic molecules.
 Source: studylib.net
Source: studylib.net
Water has high dielectric constant. These properties include solvency cohesion and adhesion high surface temper. Water has high dielectric constant. These two unusual properties allow water to moderate earth s. Learn about its physical chemical properties of water its importance for the existence of life.
 Source: studylib.net
Source: studylib.net
Because of the polarity of the molecules water molecules are attracted to each other. Because of this in earthworm water acts as hydro skeleton. These properties include solvency cohesion and adhesion high surface temper. Cohesion high specific heat high heat of vaporization lower density of ice and high polarity. Properties of water include its chemical formula h2o density melting boiling point how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Because of this water acts as powerful solvent for salts and many non ionizable organic molecules. These properties include solvency cohesion and adhesion high surface temper. Because of this in earthworm water acts as hydro skeleton. 5 properties of water. Water s high surface tension is due to the hydrogen bonding in water molecules.
 Source: edu.glogster.com
Source: edu.glogster.com
It helps organisms to tolerate pressure and compression. Water has very strong intermolecular forces hence the low vapor pressure but it s even lower compared to larger molecules with low vapor pressures. Cohesion is what we call the ability of water to attract other water molecules. Properties of water include its chemical formula h2o density melting boiling point how one molecule of water has two hydrogen atoms covalently bonded to a one oxygen atom. This opposes the attraction of opposite charges of ions.
 Source: m.youtube.com
Source: m.youtube.com
Cohesion is a key property of water. These molecules are held together by the hydrogen bonds in water. This four minute animation describes the properties of water that support life. Because of the polarity of the molecules water molecules are attracted to each other. Because of this water acts as powerful solvent for salts and many non ionizable organic molecules.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Because of this water acts as powerful solvent for salts and many non ionizable organic molecules. Water has very strong intermolecular forces hence the low vapor pressure but it s even lower compared to larger molecules with low vapor pressures. Cohesion is a key property of water. 5 properties of water. It helps organisms to tolerate pressure and compression.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title name the properties of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.