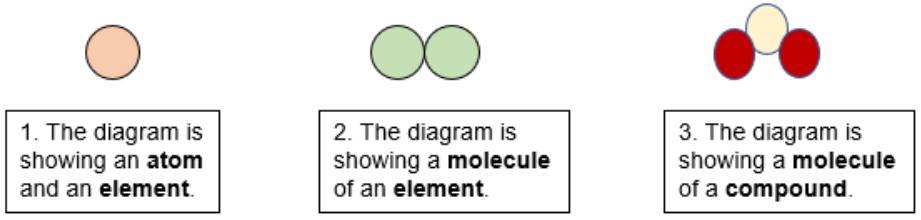
Nacl in water equation
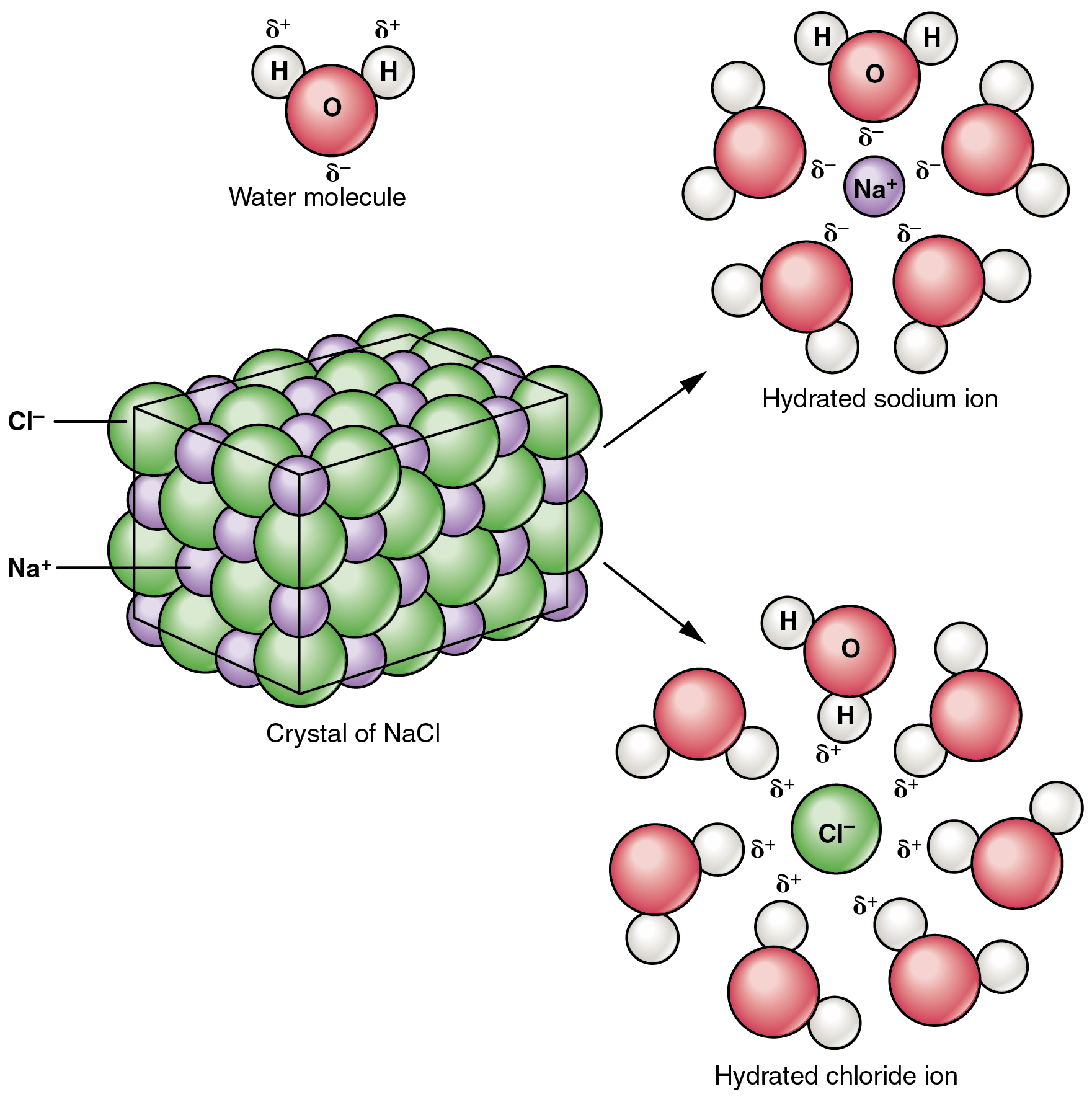
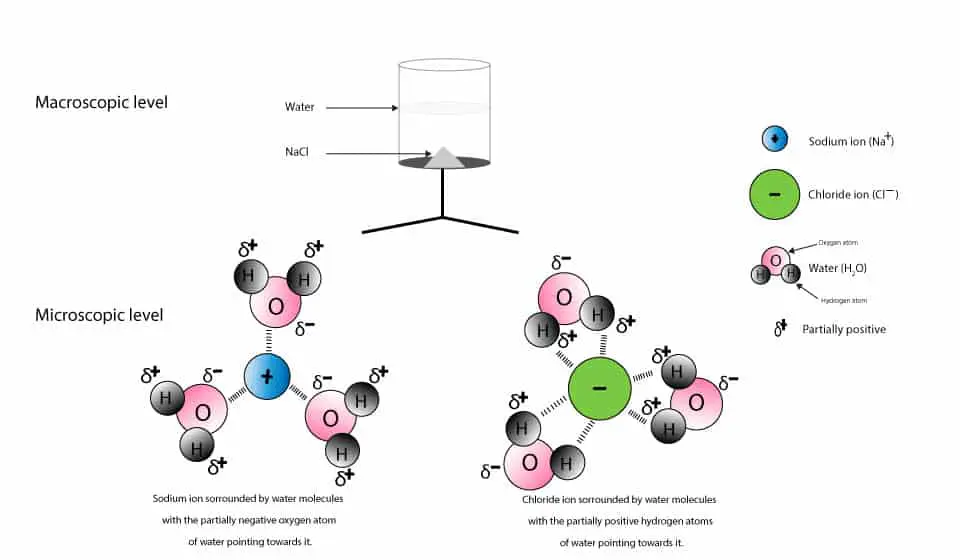
Nacl In Water Equation. This surrounding of sodium and chloride ions by water molecules is called hydration. Therefore when we write na aq or cl aq the symbol aq aqueous usually means that each ion is attracted to and surrounded by several water molecules. In the case of sodium chloride nacl nacl for example the positive sodium ions na na are attracted to the negative pole of the water molecule while the negative chloride ions cl cl are attracted to the positive. Thus the ions are solvated hydrated.
 Oneclass Write The Dissociation Equations For The Dissolutions Of Nacl S Cacl2 S And Nh4no3 S In From oneclass.com
Oneclass Write The Dissociation Equations For The Dissolutions Of Nacl S Cacl2 S And Nh4no3 S In From oneclass.com
How sodium chloride dissolves. With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl. Thus the ions are solvated hydrated. Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. Therefore when we write na aq or cl aq the symbol aq aqueous usually means that each ion is attracted to and surrounded by several water molecules. Water molecules are electrically neutral but their geometry causes them to be polarized meaning that the positive and negative charges are positioned in such a way as to be opposite one another.
In the case of sodium chloride nacl nacl for example the positive sodium ions na na are attracted to the negative pole of the water molecule while the negative chloride ions cl cl are attracted to the positive.
Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. It is the polar nature of water that allows ionic compounds to dissolve in it. In the case of sodium chloride nacl nacl for example the positive sodium ions na na are attracted to the negative pole of the water molecule while the negative chloride ions cl cl are attracted to the positive. Dissociation of sodium chloride in water. Sodium chloride ˌsoʊdiəm ˈklɔːraɪd commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula nacl representing a 1 1 ratio of sodium and chloride ions. How sodium chloride dissolves.
 Source: youtube.com
Source: youtube.com
Dissociation of sodium chloride in water. Okay first of all nacl better known as table salt does not react with water so the equation would be. It is the polar nature of water that allows ionic compounds to dissolve in it. Water molecules are electrically neutral but their geometry causes them to be polarized meaning that the positive and negative charges are positioned in such a way as to be opposite one another. How sodium chloride dissolves.
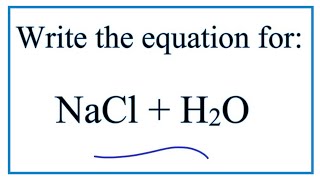 Source: youtube.com
Source: youtube.com
Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. Now when agno3 is added it will react with. It is the polar nature of water that allows ionic compounds to dissolve in it. Thus the ions are solvated hydrated. With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl.
 Source: pinterest.com
Source: pinterest.com
Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. Therefore when we write na aq or cl aq the symbol aq aqueous usually means that each ion is attracted to and surrounded by several water molecules. Water molecules are electrically neutral but their geometry causes them to be polarized meaning that the positive and negative charges are positioned in such a way as to be opposite one another. Okay first of all nacl better known as table salt does not react with water so the equation would be. In the case of sodium chloride nacl nacl for example the positive sodium ions na na are attracted to the negative pole of the water molecule while the negative chloride ions cl cl are attracted to the positive.
 Source: oneclass.com
Source: oneclass.com
With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl. Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. Water molecules are electrically neutral but their geometry causes them to be polarized meaning that the positive and negative charges are positioned in such a way as to be opposite one another. Therefore when we write na aq or cl aq the symbol aq aqueous usually means that each ion is attracted to and surrounded by several water molecules. With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl.
 Source: socratic.org
Source: socratic.org
Thus the ions are solvated hydrated. With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl. Nacl s h2o na aq cl aq h2o. Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. It is the polar nature of water that allows ionic compounds to dissolve in it.

Water molecules are electrically neutral but their geometry causes them to be polarized meaning that the positive and negative charges are positioned in such a way as to be opposite one another. This surrounding of sodium and chloride ions by water molecules is called hydration. It is the polar nature of water that allows ionic compounds to dissolve in it. Dissociation of sodium chloride in water. Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction.
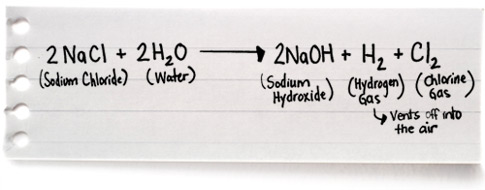 Source: thepoolshoppe.ca
Source: thepoolshoppe.ca
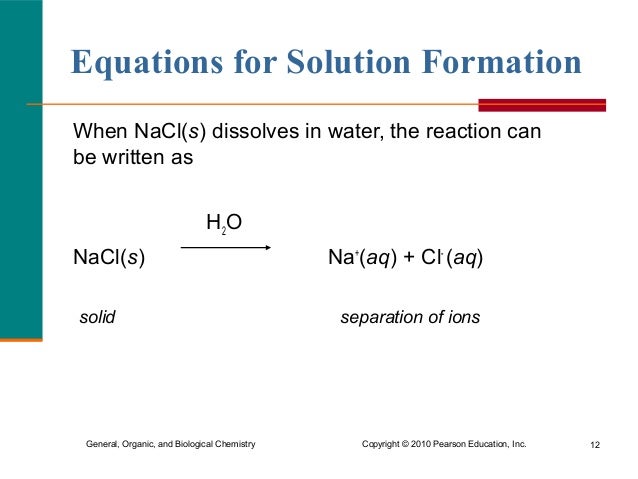
Nacl s h2o na aq cl aq h2o. Thus the ions are solvated hydrated. How sodium chloride dissolves. Now when agno3 is added it will react with. It is the polar nature of water that allows ionic compounds to dissolve in it.
 Source: youtube.com
Source: youtube.com
Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl. It is the polar nature of water that allows ionic compounds to dissolve in it. This surrounding of sodium and chloride ions by water molecules is called hydration. Okay first of all nacl better known as table salt does not react with water so the equation would be.
 Source: khanacademy.org
Source: khanacademy.org
Therefore when we write na aq or cl aq the symbol aq aqueous usually means that each ion is attracted to and surrounded by several water molecules. Water molecules are electrically neutral but their geometry causes them to be polarized meaning that the positive and negative charges are positioned in such a way as to be opposite one another. With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl. Therefore when we write na aq or cl aq the symbol aq aqueous usually means that each ion is attracted to and surrounded by several water molecules. Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Now when agno3 is added it will react with. With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl. Dissociation of sodium chloride in water. It is the polar nature of water that allows ionic compounds to dissolve in it. How sodium chloride dissolves.
 Source: slideplayer.com
Source: slideplayer.com
Okay first of all nacl better known as table salt does not react with water so the equation would be. Dissociation of sodium chloride in water. Water molecules are electrically neutral but their geometry causes them to be polarized meaning that the positive and negative charges are positioned in such a way as to be opposite one another. This surrounding of sodium and chloride ions by water molecules is called hydration. With molar masses of 22 99 and 35 45 g mol respectively 100 g of nacl contains 39 34 g na and 60 66 g cl.
 Source: quora.com
Source: quora.com
It is the polar nature of water that allows ionic compounds to dissolve in it. Okay first of all nacl better known as table salt does not react with water so the equation would be. This surrounding of sodium and chloride ions by water molecules is called hydration. Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. It is the polar nature of water that allows ionic compounds to dissolve in it.
Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. Now when agno3 is added it will react with. Therefore when we write na aq or cl aq the symbol aq aqueous usually means that each ion is attracted to and surrounded by several water molecules. Nacl s h2o na aq cl aq h2o. This surrounding of sodium and chloride ions by water molecules is called hydration.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
It is the polar nature of water that allows ionic compounds to dissolve in it. Now when agno3 is added it will react with. Water molecules are electrically neutral but their geometry causes them to be polarized meaning that the positive and negative charges are positioned in such a way as to be opposite one another. In the case of sodium chloride nacl nacl for example the positive sodium ions na na are attracted to the negative pole of the water molecule while the negative chloride ions cl cl are attracted to the positive. Sodium chloride ˌsoʊdiəm ˈklɔːraɪd commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula nacl representing a 1 1 ratio of sodium and chloride ions.
 Source: slideshare.net
Source: slideshare.net
In the case of sodium chloride nacl nacl for example the positive sodium ions na na are attracted to the negative pole of the water molecule while the negative chloride ions cl cl are attracted to the positive. Sodium chloride nacl is in fact the joining of an na ion and a cl ion which mutually attract one another via electrostatic attraction. This surrounding of sodium and chloride ions by water molecules is called hydration. Dissociation of sodium chloride in water. Now when agno3 is added it will react with.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title nacl in water equation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.