Molecular formula for oxygen gas
Molecular Formula For Oxygen Gas. The chemical symbol for the element oxygen is o. It is an allotrope of oxygen that is much less stable than the diatomic allotrope o 2 breaking down in the lower atmosphere to o 2 ozone is formed from dioxygen by the action of ultraviolet uv light and electrical discharges within the. The chemical formula for oxygen in its elemental form oxygen gas is o2. The molecular formula for oxygen gas is o 2.
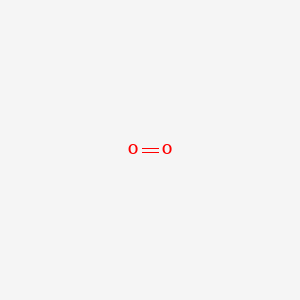
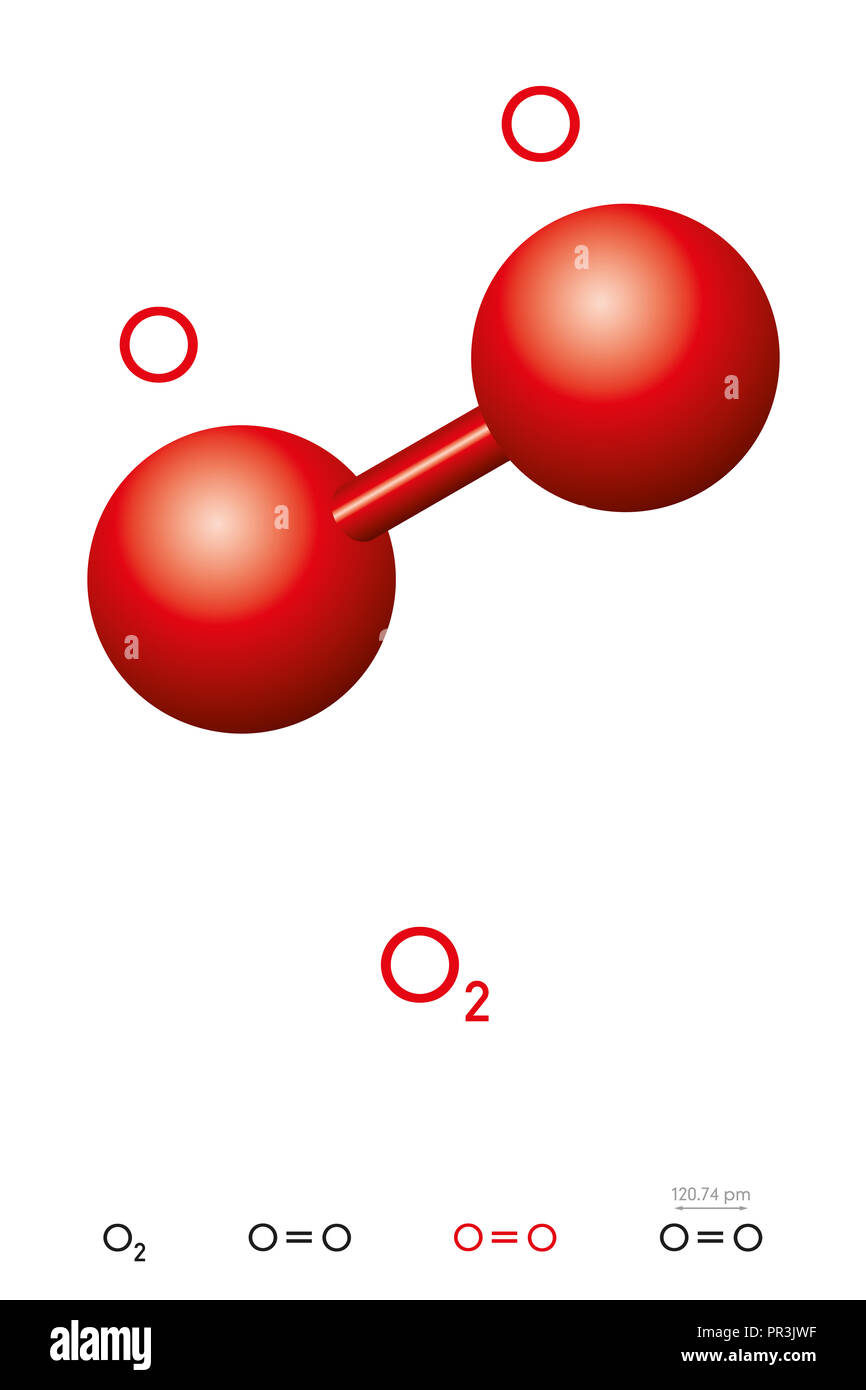
Monoisotopic mass 31 989828 da. It is an allotrope of oxygen that is much less stable than the diatomic allotrope o 2 breaking down in the lower atmosphere to o 2 ozone is formed from dioxygen by the action of ultraviolet uv light and electrical discharges within the. Molecular formula o 2. Oxygen is the chemical element with the symbol o and atomic number 8. Oxygen o2 2 pubchem. Ozone ˈ oʊ z oʊ n or trioxygen is an inorganic molecule with the chemical formula o 3 it is a pale blue gas with a distinctively pungent smell.
It is an allotrope of oxygen that is much less stable than the diatomic allotrope o 2 breaking down in the lower atmosphere to o 2 ozone is formed from dioxygen by the action of ultraviolet uv light and electrical discharges within the.
An oxygen atom has 6 electrons in its outer shell. Oxygen o2 2 pubchem. Oxygen is in group 6 of the periodic table. 2 atoms as ions are 2 x o 2. Molecular oxygen is essential for life as it is used for respiration by many organisms. These become covalently bonded to form the diatomic molecule of o2.
 Source: quora.com
Source: quora.com
O atomic mass. It s also essential for fossil fuel combustion. Molecular oxygen is essential for life as it is used for respiration by many organisms. Co valent means shared valence electrons. Average mass 31 999 da.
 Source: socratic.org
Source: socratic.org
There are no home runs or bonds formed with 4 pairs of electrons. Oxygen has a valence of 2. It is an allotrope of oxygen that is much less stable than the diatomic allotrope o 2 breaking down in the lower atmosphere to o 2 ozone is formed from dioxygen by the action of ultraviolet uv light and electrical discharges within the. Average mass 31 999 da. Monoisotopic mass 31 989828 da.
 Source: socratic.org
Source: socratic.org
It s also essential for fossil fuel combustion. The chemical symbol for the element oxygen is o. In addition the atoms do not have to be from the same element when forming covalent bonds. Oxygen is in group 6 of the periodic table. It s also essential for fossil fuel combustion.
 Source: alamy.com
Source: alamy.com
Oxygen is the chemical element with the symbol o and atomic number 8. An example of a triple covalent bond occurs when two nitrogen atoms share their 3 pairs of electrons. Oxygen o2 2 pubchem. The chemical formula for oxygen in its elemental form oxygen gas is o2. Co valent means shared valence electrons.
 Source: chegg.com
Source: chegg.com
The chemical formula for oxygen in its elemental form oxygen gas is o2. Monoisotopic mass 31 989828 da. It is an allotrope of oxygen that is much less stable than the diatomic allotrope o 2 breaking down in the lower atmosphere to o 2 ozone is formed from dioxygen by the action of ultraviolet uv light and electrical discharges within the. In addition the atoms do not have to be from the same element when forming covalent bonds. There are no home runs or bonds formed with 4 pairs of electrons.
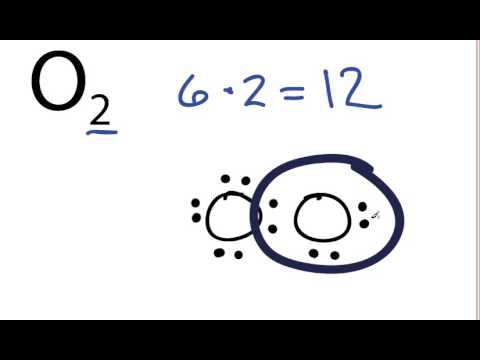 Source: m.youtube.com
Source: m.youtube.com
Oxygen has a valence of 2. There are no home runs or bonds formed with 4 pairs of electrons. Monoisotopic mass 31 989828 da. Molecular oxygen o 2 is a diatomic molecule that is composed of two oxygen atoms held together by a covalent bond. 2 atoms as ions are 2 x o 2.
 Source: youtube.com
Source: youtube.com
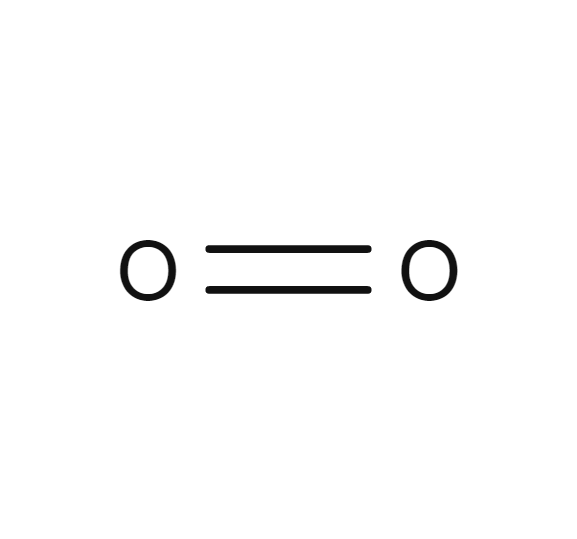
Oxygen o2 2 pubchem. Two oxygen atoms will each share two electrons to form two covalent bonds and make an oxygen molecule o 2. Co valent means shared valence electrons. At standard temperature and pressure two. In addition the atoms do not have to be from the same element when forming covalent bonds.
 Source: chegg.com
Source: chegg.com
There are no home runs or bonds formed with 4 pairs of electrons. Oxygen o2 2 pubchem. It is an allotrope of oxygen that is much less stable than the diatomic allotrope o 2 breaking down in the lower atmosphere to o 2 ozone is formed from dioxygen by the action of ultraviolet uv light and electrical discharges within the. It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compounds after hydrogen and helium oxygen is the third most abundant element in the universe by mass. Monoisotopic mass 31 989828 da.
 Source: youtube.com
Source: youtube.com
An example of a triple covalent bond occurs when two nitrogen atoms share their 3 pairs of electrons. Two oxygen atoms will each share two electrons to form two covalent bonds and make an oxygen molecule o 2. Monoisotopic mass 31 989828 da. Molecular formula o 2. The chemical formula for oxygen in its elemental form oxygen gas is o2.
 Source: encyclopedia.airliquide.com
Source: encyclopedia.airliquide.com
Oxygen is in group 6 of the periodic table. The molecular formula for oxygen gas is o 2. Molecular oxygen o 2 is a diatomic molecule that is composed of two oxygen atoms held together by a covalent bond. Ozone ˈ oʊ z oʊ n or trioxygen is an inorganic molecule with the chemical formula o 3 it is a pale blue gas with a distinctively pungent smell. An example of a triple covalent bond occurs when two nitrogen atoms share their 3 pairs of electrons.
 Source: britannica.com
Source: britannica.com
Ozone ˈ oʊ z oʊ n or trioxygen is an inorganic molecule with the chemical formula o 3 it is a pale blue gas with a distinctively pungent smell. An oxygen atom has 6 electrons in its outer shell. At standard temperature and pressure two. It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compounds after hydrogen and helium oxygen is the third most abundant element in the universe by mass. Ozone ˈ oʊ z oʊ n or trioxygen is an inorganic molecule with the chemical formula o 3 it is a pale blue gas with a distinctively pungent smell.
 Source: mikeblaber.org
Source: mikeblaber.org
At standard temperature and pressure two. The chemical formula for oxygen in its elemental form oxygen gas is o2. Monoisotopic mass 31 989828 da. It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compounds after hydrogen and helium oxygen is the third most abundant element in the universe by mass. An example of a triple covalent bond occurs when two nitrogen atoms share their 3 pairs of electrons.
 Source: mikeblaber.org
Source: mikeblaber.org
In addition the atoms do not have to be from the same element when forming covalent bonds. Oxygen has a valence of 2. O atomic mass. Two oxygen atoms will each share two electrons to form two covalent bonds and make an oxygen molecule o 2. 2 atoms as ions are 2 x o 2.
Source: chemspider.com
At standard temperature and pressure two. Molecular formula o 2. At standard temperature and pressure two. Molecular oxygen o 2 is a diatomic molecule that is composed of two oxygen atoms held together by a covalent bond. The chemical symbol for the element oxygen is o.

These become covalently bonded to form the diatomic molecule of o2. Molecular oxygen o 2 is a diatomic molecule that is composed of two oxygen atoms held together by a covalent bond. Oxygen o2 2 pubchem. It s also essential for fossil fuel combustion. The chemical symbol for the element oxygen is o.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molecular formula for oxygen gas by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





