Metals in science
Metals In Science. This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides. Bronze is an alloy of copper and tin while brass is the alloy you get when you mix copper and zinc. Alkaline earth metals are found in compounds with many different minerals. The periodic table of metals and nonmetals can be broken down to give you a sense of each element s characteristics.
 Is Matter Around Us Pure Class 9 Science Chapter 2 From takshilalearning.com
Is Matter Around Us Pure Class 9 Science Chapter 2 From takshilalearning.com
The periodic table of metals and nonmetals can be broken down to give you a sense of each element s characteristics. The most abundant varieties in the earth s crust are aluminum iron calcium sodium potassium and magnesium. The definition of metal. The periodic table contains a lot of useful information on the elements. In chemistry a metal is an element that readily forms positive ions cations and has metallic bonds. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc.
A substance with high electrical conductivity luster and malleability which readily loses electrons to form positive ions cations.
The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. This group includes calcium magnesium and barium. This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides. Approximately three quarters of all known chemical elements are metals. The periodic table contains a lot of useful information on the elements. The definition of metal.
 Source: youtube.com
Source: youtube.com
In chemistry a metal is an element that readily forms positive ions cations and has metallic bonds. The metals share several common properties including. Most elements can be considered metals. The periodic table of metals and nonmetals can be broken down to give you a sense of each element s characteristics. In chemistry a metal is an element that readily forms positive ions cations and has metallic bonds.
 Source: science.olympiadsuccess.com
Source: science.olympiadsuccess.com
The most abundant varieties in the earth s crust are aluminum iron calcium sodium potassium and magnesium. The most common definition includes niobium molybdenum tantalum tungsten and rhenium. Alkaline earth metals are found in compounds with many different minerals. The most abundant varieties in the earth s crust are aluminum iron calcium sodium potassium and magnesium. Most elements are metals.
 Source: science.sciencemag.org
Source: science.sciencemag.org
Approximately three quarters of all known chemical elements are metals. Metals are otherwise defined according to their position on the periodic table. The most abundant varieties in the earth s crust are aluminum iron calcium sodium potassium and magnesium. This group includes calcium magnesium and barium. Metals nonmetals and metalloids make up the periodic table with metals constituting the large majority of all metals.

Most elements can be considered metals. In chemistry a metal is an element that readily forms positive ions cations and has metallic bonds. The metals share several common properties including. Most elements are metals. Steel is an alloy of iron for example that contains a small amount of carbon different types of steel contain more or less carbon.
 Source: slideshare.net
Source: slideshare.net
The definition of metal. This group includes calcium magnesium and barium. Most elements are metals. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides.
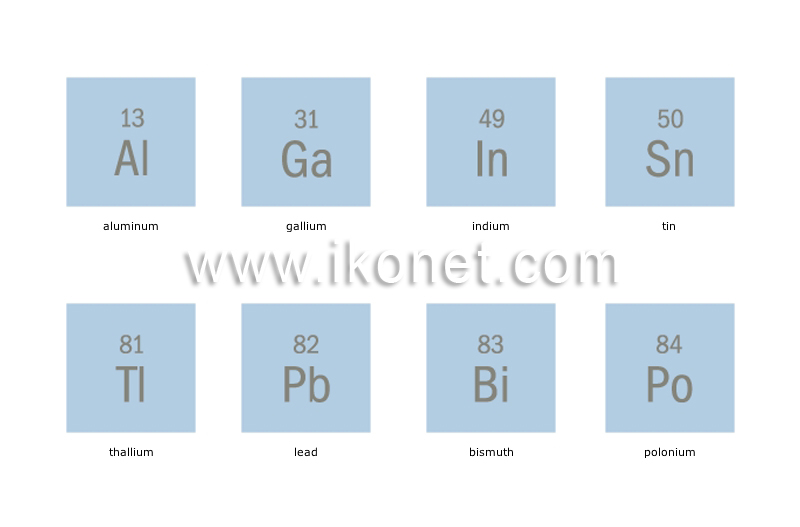 Source: ikonet.com
Source: ikonet.com
This group includes calcium magnesium and barium. The periodic table of metals and nonmetals can be broken down to give you a sense of each element s characteristics. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. They are less reactive than alkali metals as well as harder and have higher melting points. Metal groupings on the periodic table.
 Source: youtube.com
Source: youtube.com
In materials science metallurgy and engineering a refractory metal is a metal that is extraordinarily resistant to heat and wear. Metals are sometimes described as a lattice of positive ions surrounded by a cloud of. Metals are otherwise defined according to their position on the periodic table. Most elements can be considered metals. Which metals belong to this category varies.
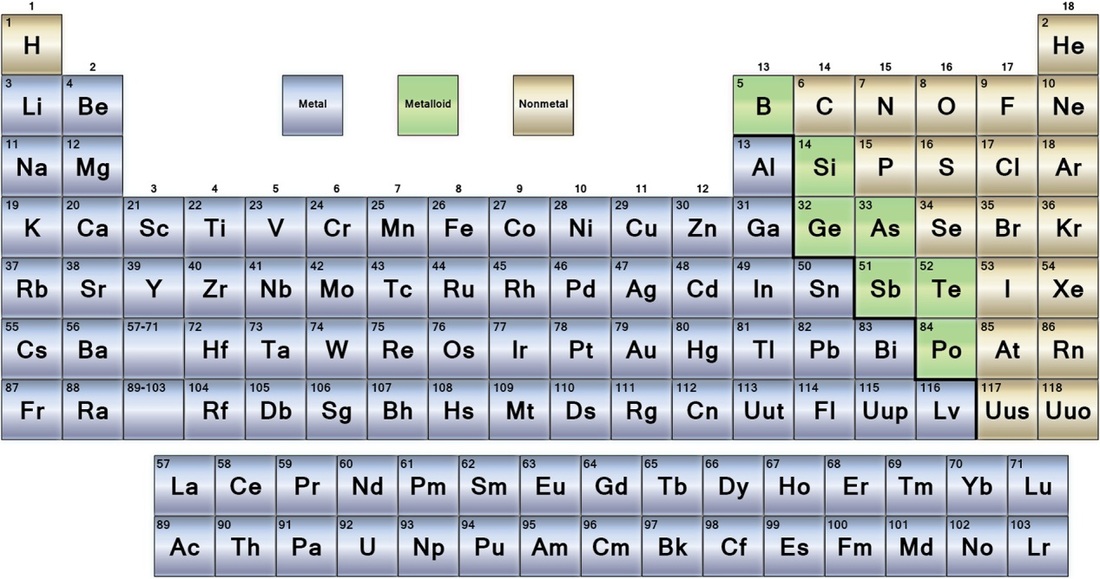 Source: wghsjuniorscience.weebly.com
Source: wghsjuniorscience.weebly.com
Bronze is an alloy of copper and tin while brass is the alloy you get when you mix copper and zinc. This group includes calcium magnesium and barium. This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides. The metals share several common properties including. The periodic table contains a lot of useful information on the elements.
 Source: pinterest.com
Source: pinterest.com
The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. In chemistry a metal is an element that readily forms positive ions cations and has metallic bonds. Approximately three quarters of all known chemical elements are metals. The definition of metal. They are grouped together in the middle to the left hand side of the periodic table.
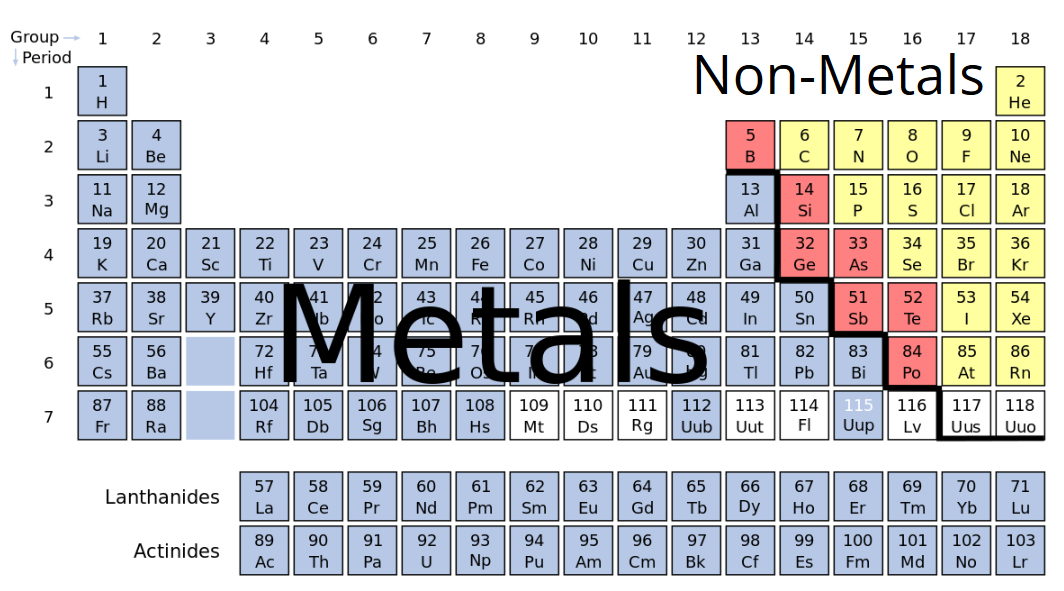 Source: sciencetrends.com
Source: sciencetrends.com
This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides. The definition of metal. They are less reactive than alkali metals as well as harder and have higher melting points. Using it you should be able to classify all the elements in different ways. This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides.
 Source: pinterest.com
Source: pinterest.com
A substance with high electrical conductivity luster and malleability which readily loses electrons to form positive ions cations. Bronze is an alloy of copper and tin while brass is the alloy you get when you mix copper and zinc. In materials science metallurgy and engineering a refractory metal is a metal that is extraordinarily resistant to heat and wear. Approximately three quarters of all known chemical elements are metals. The most abundant varieties in the earth s crust are aluminum iron calcium sodium potassium and magnesium.

In materials science metallurgy and engineering a refractory metal is a metal that is extraordinarily resistant to heat and wear. Most elements can be considered metals. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. The periodic table contains a lot of useful information on the elements. They are less reactive than alkali metals as well as harder and have higher melting points.
 Source: schooltutoring.com
Source: schooltutoring.com
They are grouped together in the middle to the left hand side of the periodic table. Metals are sometimes described as a lattice of positive ions surrounded by a cloud of. In chemistry a metal is an element that readily forms positive ions cations and has metallic bonds. The definition of metal. Most elements can be considered metals.
 Source: takshilalearning.com
Source: takshilalearning.com
Steel is an alloy of iron for example that contains a small amount of carbon different types of steel contain more or less carbon. The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. Metals are sometimes described as a lattice of positive ions surrounded by a cloud of. The metals share several common properties including. Most elements can be considered metals.
 Source: steemit.com
Source: steemit.com
Potassium and sodium are two alkali metals. Approximately three quarters of all known chemical elements are metals. The most abundant varieties in the earth s crust are aluminum iron calcium sodium potassium and magnesium. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. The most common definition includes niobium molybdenum tantalum tungsten and rhenium.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title metals in science by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





