Metals are good conductors of electricity because
Metals Are Good Conductors Of Electricity Because. This is due to their high electropositive character. Metals are good conductor of electricity because they contain free electrons. Pure metals on the other hand are superlative conductors because although their atoms are restrained by structure their electrons are not they re free. The electrons are unrestrained and therefore begin to move around haphazardly when the source imparts kinetic energy into them.
 Activity 3 6 Ncert Class 10 Science Metals And Non Metals Studdy From studdy.org
Activity 3 6 Ncert Class 10 Science Metals And Non Metals Studdy From studdy.org
Solution by examveda team metals are good conductors of electricity because they contain free electrons. Metals are good conductors of electricity because electrons can flow freely in them. Physics section 1. Start typing press enter or esc to close. They contain free electrons b. Metals are good conductors of electricity because a.
Find the maximum velocity for the overturn of a car moving on a circular track of radius 100 m.
The electrons are unrestrained and therefore begin to move around haphazardly when the source imparts kinetic energy into them. They contain free electrons b. The co efficient of friction between the road and tyre is 0 2. Metals are good conductor of electricity because they contain free electrons. Solution by examveda team metals are good conductors of electricity because they contain free electrons. This is because silver is more expensive than copper.
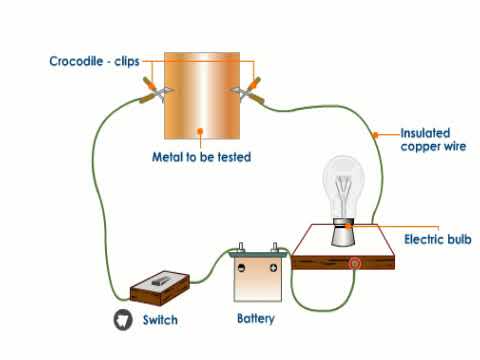 Source: youtube.com
Source: youtube.com
Pure metals on the other hand are superlative conductors because although their atoms are restrained by structure their electrons are not they re free. This is due to their high electropositive character. When it comes to the use of metals for electrical applications copper is more widely used than silver. Physics section 1. Solution by examveda team metals are good conductors of electricity because they contain free electrons.
 Source: chegg.com
Source: chegg.com
The atomic structure of metals is unique because of the presence of one two or three valence electrons in the outer orbit of the atoms and this is true for all metallic elements. Find the maximum velocity for the overturn of a car moving on a circular track of radius 100 m. The electrons are unrestrained and therefore begin to move around haphazardly when the source imparts kinetic energy into them. The co efficient of friction between the road and tyre is 0 2. Metals are good conductors of electricity because.
 Source: slideplayer.com
Source: slideplayer.com
Metals are good conductor of electricity because they contain free electrons. Metals are good conductor of electricity because they contain free electrons. They contain free electrons b. This is due to their high electropositive character. Alkali and alkaline earth metals react with water to produce hydrogen gas and metal hydroxides.
 Source: slideplayer.com
Source: slideplayer.com
Metals are good conductors of electricity because. Metals are good conductors of electricity because a. The atoms are lightly packed c. Silver is considered to be the best conductor of electricity followed by copper and then gold. Metals are good conductors of electricity because.
 Source: science4fun.info
Source: science4fun.info
Metals are good conductors of electricity because a. Silver is considered to be the best conductor of electricity followed by copper and then gold. They contain free electrons b. The atoms are lightly packed c. Metals are good conductors of electricity because a.
 Source: toppr.com
Source: toppr.com
Metals are good conductors of electricity because. Metals are good conductors of electricity because. This is due to their high electropositive character. Metals are good conductors of electricity because electrons can flow freely in them. They contain free electrons b.

Metals are good conductors of electricity because. They contain free electrons b. Silver is considered to be the best conductor of electricity followed by copper and then gold. The atomic structure of metals is unique because of the presence of one two or three valence electrons in the outer orbit of the atoms and this is true for all metallic elements. This is due to their high electropositive character.
 Source: brainly.in
Source: brainly.in
This is due to their high electropositive character. Pure metals on the other hand are superlative conductors because although their atoms are restrained by structure their electrons are not they re free. The atomic structure of metals is unique because of the presence of one two or three valence electrons in the outer orbit of the atoms and this is true for all metallic elements. Metals are good conductors of electricity because electrons can flow freely in them. Solution by examveda team metals are good conductors of electricity because they contain free electrons.
 Source: studdy.org
Source: studdy.org
Metals are good conductors of electricity because electrons can flow freely in them. Metals are good conductors of electricity because. Pure metals on the other hand are superlative conductors because although their atoms are restrained by structure their electrons are not they re free. When it comes to the use of metals for electrical applications copper is more widely used than silver. This is due to their high electropositive character.
 Source: toppr.com
Source: toppr.com
This is because silver is more expensive than copper. This is due to their high electropositive character. The atomic structure of metals is unique because of the presence of one two or three valence electrons in the outer orbit of the atoms and this is true for all metallic elements. Pure metals on the other hand are superlative conductors because although their atoms are restrained by structure their electrons are not they re free. Metals are good conductors of electricity because a.
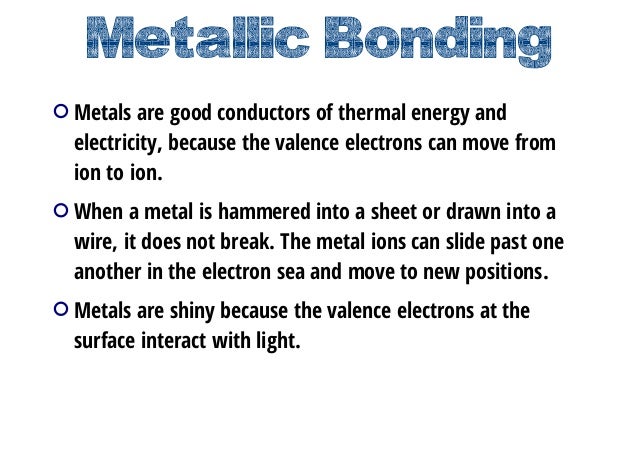 Source: slideshare.net
Source: slideshare.net
When it comes to the use of metals for electrical applications copper is more widely used than silver. Metals are good conductors of electricity because. Metals are good conductor of electricity because they contain free electrons. When it comes to the use of metals for electrical applications copper is more widely used than silver. The atomic structure of metals is unique because of the presence of one two or three valence electrons in the outer orbit of the atoms and this is true for all metallic elements.
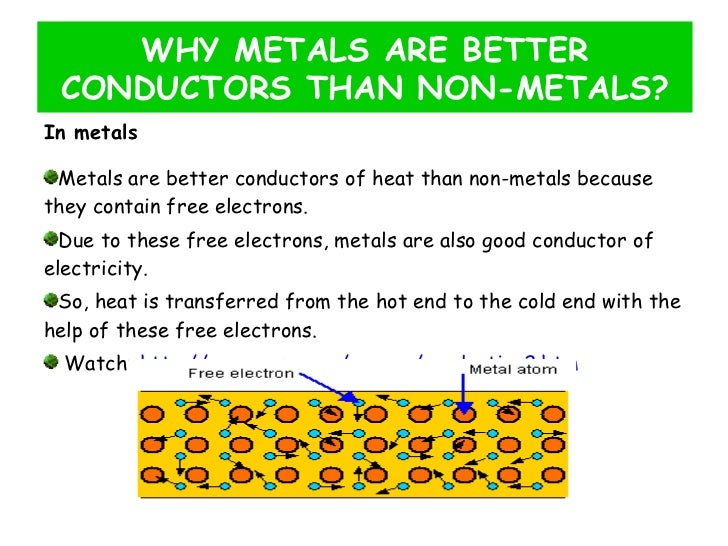 Source: pt.slideshare.net
Source: pt.slideshare.net
Metals are good conductors of electricity because. Metals are good conductors of electricity because electrons can flow freely in them. The atoms are lightly packed c. They contain free electrons b. When it comes to the use of metals for electrical applications copper is more widely used than silver.
 Source: slideplayer.com
Source: slideplayer.com
This is because silver is more expensive than copper. Metals are good conductor of electricity because they contain free electrons. Find the maximum velocity for the overturn of a car moving on a circular track of radius 100 m. Physics section 1. Alkali and alkaline earth metals react with water to produce hydrogen gas and metal hydroxides.
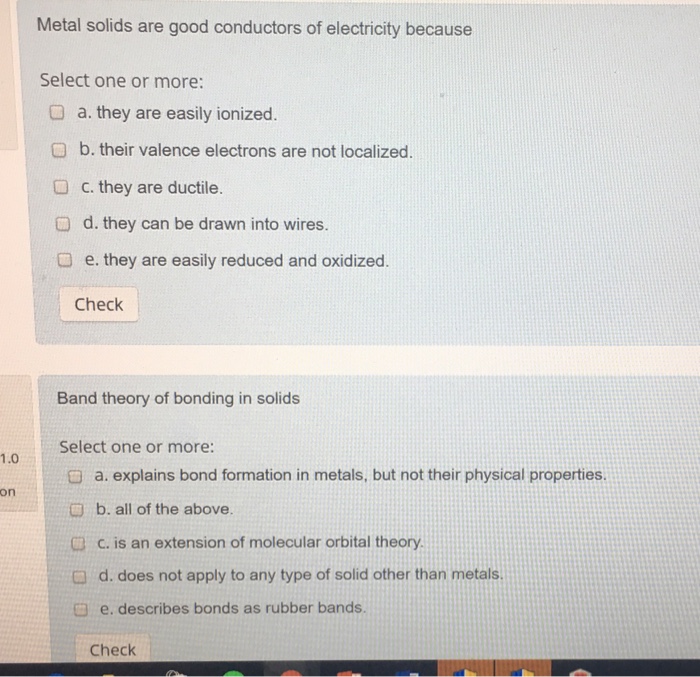
The electrons are unrestrained and therefore begin to move around haphazardly when the source imparts kinetic energy into them. Start typing press enter or esc to close. Metals are good conductors of electricity because. They contain free electrons b. The co efficient of friction between the road and tyre is 0 2.
 Source: quora.com
Source: quora.com
Metals are good conductors of electricity because. This is because silver is more expensive than copper. This is due to their high electropositive character. Silver is considered to be the best conductor of electricity followed by copper and then gold. Start typing press enter or esc to close.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title metals are good conductors of electricity because by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.




