Metallic element list
Metallic Element List. Although separate on the periodic table lanthanides and actinides are really specific types of transition metals. Metal elements are bright because the mobile electrons are delocalized. Most elements can be considered metals. In the periodic table metals are separated into the groups detailed in the following list.
 What Are The Parts Of The Periodic Table From thoughtco.com
What Are The Parts Of The Periodic Table From thoughtco.com
Moscow oblast russia where the element was first synthesised. Nearly 75 of all the elements in the periodic table are classified as metals. 7 290 13 5 700 1400 0. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. Even oxygen has a metallic form as a solid. Sometimes this element is considered to be a metalloid rather than a nonmetal.
This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides.
They have also thermal conductivity. Examples of metals are gold aluminium copper iron lead silver platinum uranium and zinc. They have also thermal conductivity. Hydrogen acts as an alkali metal under extreme pressure. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. Flerov laboratory of nuclear reactions part of jinr where the element was synthesised.
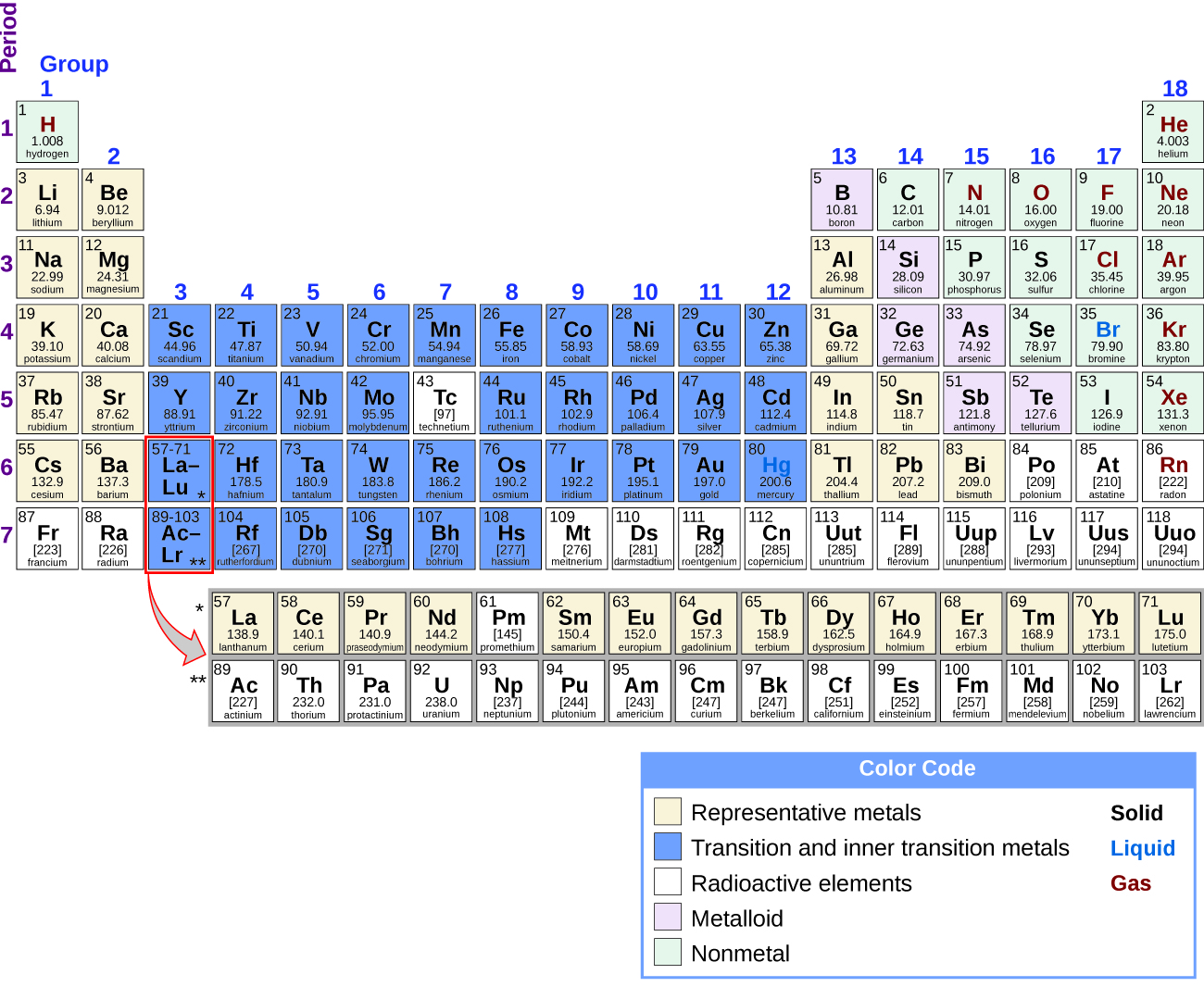 Source: chem.libretexts.org
Source: chem.libretexts.org
Hydrogen acts as an alkali metal under extreme pressure. The first chemical element is actinium and the last is zirconium. Sometimes this element is considered to be a metalloid rather than a nonmetal. Hydrogen acts as an alkali metal under extreme pressure. Please note that the elements do not show their natural relation towards each other as in the periodic system.
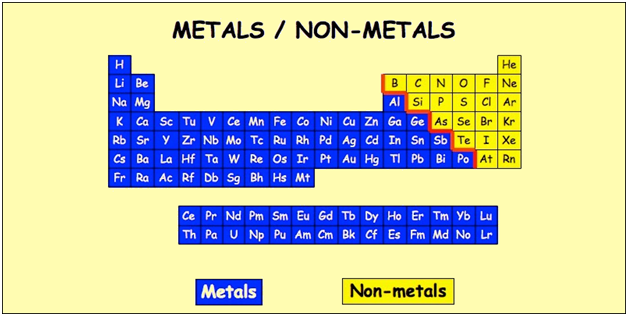 Source: periodictable.me
Source: periodictable.me
We can explain the reason of this feature again with the mobile electrons. Metal elements are bright because the mobile electrons are delocalized. Hydrogen acts as an alkali metal under extreme pressure. 7 290 13 5 700 1400 0. These bonds have an electrical conductivity.
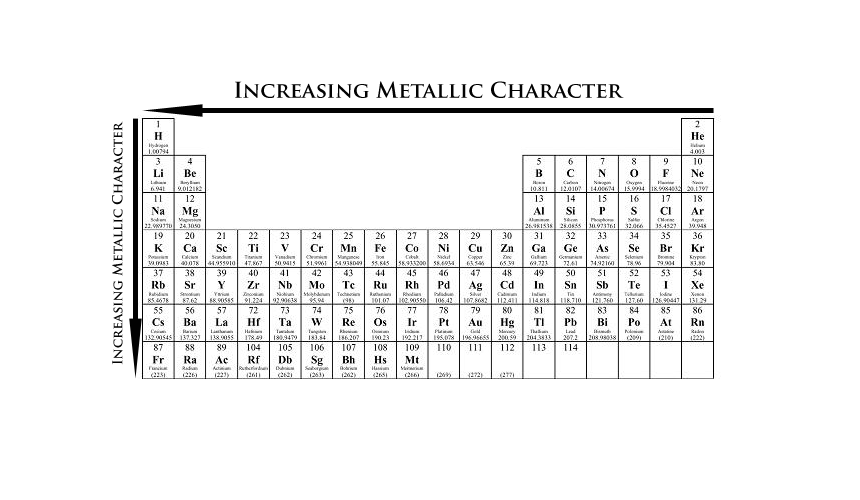 Source: 170188733453308075.weebly.com
Source: 170188733453308075.weebly.com
Metallic character isn t an all or nothing property. Flerov laboratory of nuclear reactions part of jinr where the element was synthesised. Please note that the elements do not show their natural relation towards each other as in the periodic system. Metal elements are bright because the mobile electrons are delocalized. 7 289 14 210 0.
 Source: sciencenotes.org
Source: sciencenotes.org
They are grouped together in the middle to the left hand side of the periodic table. Sometimes this element is considered to be a metalloid rather than a nonmetal. These bonds have an electrical conductivity. There you can find the metals semi conductor s non metal s inert noble gas ses halogens lanthanoides actinoids rare earth elements and transition metals. Moscow oblast russia where the element was first synthesised.
 Source: britannica.com
Source: britannica.com
Carbon for example has allotropes that behave more like metals than nonmetals. Even oxygen has a metallic form as a solid. Most elements can be considered metals. Please note that the elements do not show their natural relation towards each other as in the periodic system. The first chemical element is actinium and the last is zirconium.
 Source: ivyroses.com
Source: ivyroses.com
The first chemical element is actinium and the last is zirconium. There you can find the metals semi conductor s non metal s inert noble gas ses halogens lanthanoides actinoids rare earth elements and transition metals. This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides. The first chemical element is actinium and the last is zirconium. The properties of a metallic bond are generally explained and based on the electrons.
 Source: thoughtco.com
Source: thoughtco.com
The first chemical element is actinium and the last is zirconium. Most elements can be considered metals. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. Hydrogen acts as an alkali metal under extreme pressure. In the periodic table metals are separated into the groups detailed in the following list.
 Source: waybuilder.net
Source: waybuilder.net
Nearly 75 of all the elements in the periodic table are classified as metals. These bonds have an electrical conductivity. 7 290 13 5 700 1400 0. Flerov laboratory of nuclear reactions part of jinr where the element was synthesised. Carbon for example has allotropes that behave more like metals than nonmetals.
 Source: sciencenotes.org
Source: sciencenotes.org
Although separate on the periodic table lanthanides and actinides are really specific types of transition metals. This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides. Nearly 75 of all the elements in the periodic table are classified as metals. These bonds have an electrical conductivity. They have also thermal conductivity.
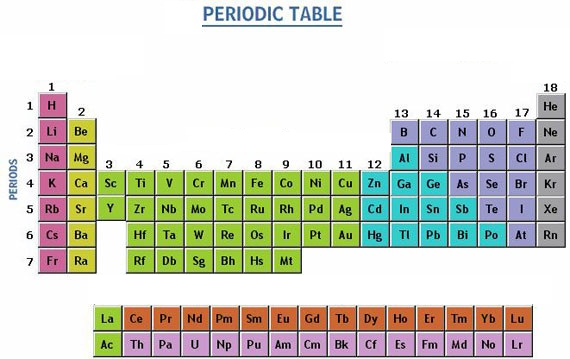 Source: periodictable.me
Source: periodictable.me
The nonmetals lis t which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. Most elements are metals. They have also thermal conductivity. The properties of a metallic bond are generally explained and based on the electrons. These bonds have an electrical conductivity.
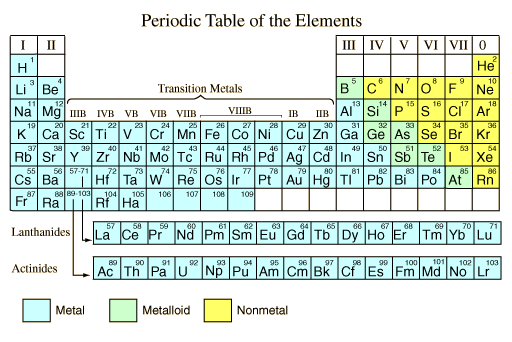 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
Hydrogen acts as an alkali metal under extreme pressure. 7 290 13 5 700 1400 0. Hydrogen acts as an alkali metal under extreme pressure. The properties of a metallic bond are generally explained and based on the electrons. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Metal elements are bright because the mobile electrons are delocalized. Metal elements are bright because the mobile electrons are delocalized. Flerov laboratory of nuclear reactions part of jinr where the element was synthesised. Although separate on the periodic table lanthanides and actinides are really specific types of transition metals. In the periodic table metals are separated into the groups detailed in the following list.
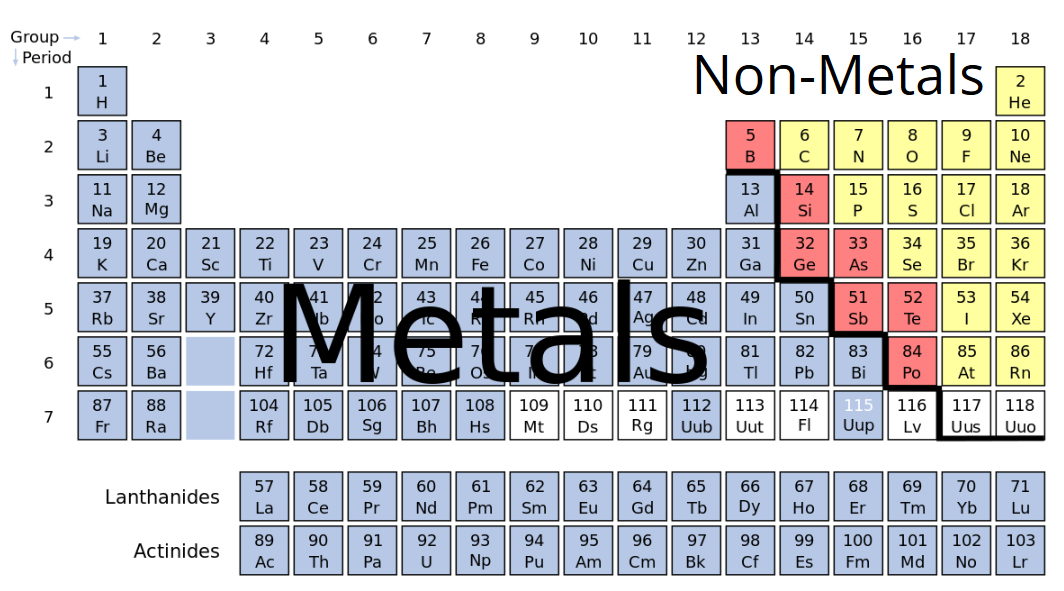 Source: sciencetrends.com
Source: sciencetrends.com
Although separate on the periodic table lanthanides and actinides are really specific types of transition metals. Examples of metals are gold aluminium copper iron lead silver platinum uranium and zinc. 7 290 13 5 700 1400 0. These bonds have an electrical conductivity. Metallic character isn t an all or nothing property.
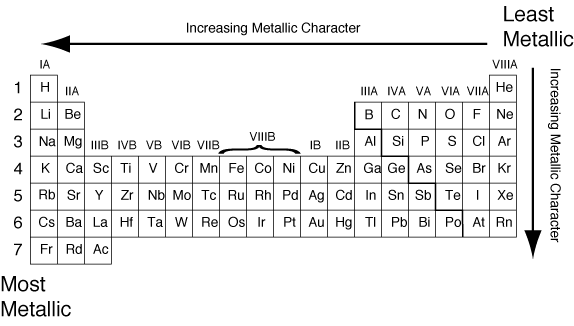 Source: socratic.org
Source: socratic.org
Metal elements are bright because the mobile electrons are delocalized. Nearly 75 of all the elements in the periodic table are classified as metals. They are grouped together in the middle to the left hand side of the periodic table. The metals list which makes up the periodic table includes iron lead gold aluminum platinum uranium zinc lithium sodium tin silver etc. Flerov laboratory of nuclear reactions part of jinr where the element was synthesised.
 Source: thoughtco.com
Source: thoughtco.com
7 290 13 5 700 1400 0. Although separate on the periodic table lanthanides and actinides are really specific types of transition metals. Even oxygen has a metallic form as a solid. Itself named after georgy flyorov russian physicist. The first chemical element is actinium and the last is zirconium.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title metallic element list by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






