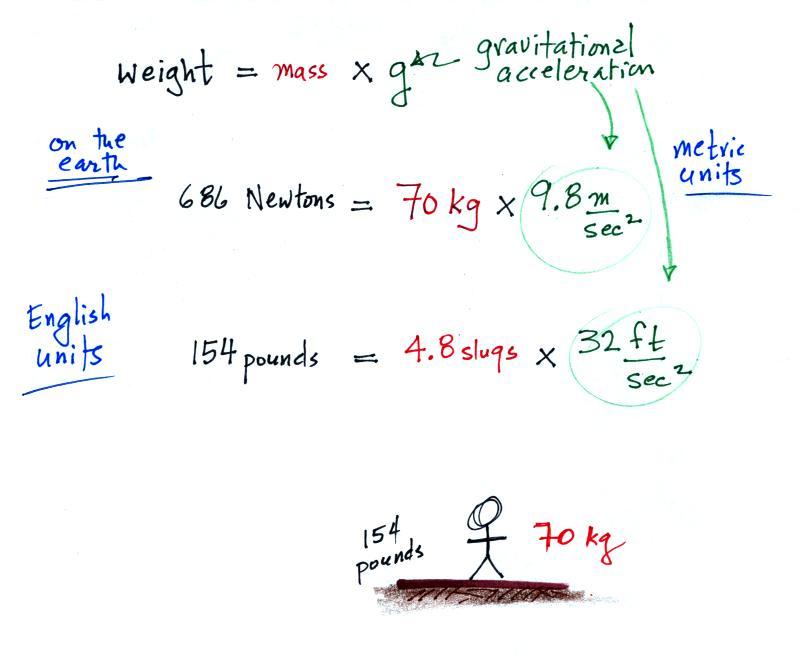
Lithium chloride flame
Lithium Chloride Flame. This process is used extensively in chemistry to determine what type and concentration of atoms a substance contains. Lithium chloride is used as a relative humidity standard in the calibration of hygrometers. As flame colorant lithium chloride is used to produce dark red flames. To create colored flame with red color safely we can use lithium chloride.
 Flame Test Experiments Involving Control Aqueous Solutions Consisting Download Scientific Diagram From researchgate.net
Flame Test Experiments Involving Control Aqueous Solutions Consisting Download Scientific Diagram From researchgate.net
Therefore to create red color we can use lithium chloride. The color is less intense than the strontium flame color. It went better than last year s demonstration i have 3 videos of those on youtube but not as g. This process is used extensively in chemistry to determine what type and concentration of atoms a substance contains. At 25 c 77 f a saturated solution 45 8 of the salt will yield an equilibrium relative humidity of 11 30. Lithium chloride is also used as a flame colorant to produce dark red flames.
Lithium may also be absorbed via the lungs.
Lithium i gives a pink flame copper ii gives a green flame and sodium i gives a yellow flame. This process is used extensively in chemistry to determine what type and concentration of atoms a substance contains. Therefore to create red color we can use lithium chloride. Lithium i gives a pink flame copper ii gives a green flame and sodium i gives a yellow flame. Serum lithium was non detectable at the first measurement whereas 0 01 0 05 mm appeared in the blood from the 1st to the 4th day. Molten licl is used for the preparation of lithium niobite graphene and carbon nanotubes.
 Source: pinterest.com
Source: pinterest.com
Lithium emmited the least energy because according to the electromagnetic spectrum dark reds oranges and yellows have a high wavelength but low energy and lithium nitrate burned a dark red. The movement of the electrons is due to a change in energy because of the reaction of the elements and the fire. The color is less intense than the strontium flame color. Lithium i gives a pink flame copper ii gives a green flame and sodium i gives a yellow flame. Therefore to create red color we can use lithium chloride.
 Source: socratic.org
Source: socratic.org
The colour of the light depends on the metal. Lithium nitrate and lithium chloride flame tests produce the same color because it is the lithium electrons that are raised to a higher energy level and then drop back down to their ground state. This process is used extensively in chemistry to determine what type and concentration of atoms a substance contains. Even though original flame has orange ish color but the natural one has unstable color and less vibrant color. Lithium chloride is also used as a flame colorant to produce dark red flames.

As flame colorant lithium chloride is used to produce dark red flames. A systemic resorption of lithium was shown in a study on 27 intensive care unit patients who were mechanically ventilated with lithium chloride coated heat and moisture exchangers for at least 5 days. To create colored flame with red color safely we can use lithium chloride. Serum lithium was non detectable at the first measurement whereas 0 01 0 05 mm appeared in the blood from the 1st to the 4th day. Molten licl is used for the preparation of lithium niobite graphene and carbon nanotubes.
 Source: fineartamerica.com
Source: fineartamerica.com
Lithium chloride belongs to salt compound with chemical formula licl. Molten licl is used for the preparation of lithium niobite graphene and carbon nanotubes. Lithium emmited the least energy because according to the electromagnetic spectrum dark reds oranges and yellows have a high wavelength but low energy and lithium nitrate burned a dark red. At 25 c 77 f a saturated solution 45 8 of the salt will yield an equilibrium relative humidity of 11 30. As flame colorant lithium chloride is used to produce dark red flames.
 Source: fineartamerica.com
Source: fineartamerica.com
Lithium nitrate and lithium chloride flame tests produce the same color because it is the lithium electrons that are raised to a higher energy level and then drop back down to their ground state. To create colored flame with red color safely we can use lithium chloride. The movement of the electrons is due to a change in energy because of the reaction of the elements and the fire. Lithium chloride imparts a red color to a flame. Lithium chloride is used as a relative humidity standard in the calibration of hygrometers.
 Source: youtube.com
Source: youtube.com
The color is less intense than the strontium flame color. A systemic resorption of lithium was shown in a study on 27 intensive care unit patients who were mechanically ventilated with lithium chloride coated heat and moisture exchangers for at least 5 days. Lithium i gives a pink flame copper ii gives a green flame and sodium i gives a yellow flame. Lithium may also be absorbed via the lungs. The color is less intense than the strontium flame color.
 Source: researchgate.net
Source: researchgate.net
A carmine red color is imparted to the flame by lithium chloride. Molten licl is used for the preparation of lithium niobite graphene and carbon nanotubes. At 25 c 77 f a saturated solution 45 8 of the salt will yield an equilibrium relative humidity of 11 30. Lithium chloride is used as a relative humidity standard in the calibration of hygrometers. Lithium chloride is also used as a flame colorant to produce dark red flames.
 Source: pinterest.com
Source: pinterest.com
Lithium chloride belongs to salt compound with chemical formula licl. Molten licl is used for the preparation of lithium niobite graphene and carbon nanotubes. Even though original flame has orange ish color but the natural one has unstable color and less vibrant color. It went better than last year s demonstration i have 3 videos of those on youtube but not as g. Lithium chloride is also used as a flame colorant to produce dark red flames.

Therefore to create red color we can use lithium chloride. Molten licl is used for the preparation of lithium niobite graphene and carbon nanotubes. This is the 08 09 school year s flame test demonstration. The color is less intense than the strontium flame color. It went better than last year s demonstration i have 3 videos of those on youtube but not as g.
 Source: youtube.com
Source: youtube.com
As flame colorant lithium chloride is used to produce dark red flames. Lithium may also be absorbed via the lungs. Lithium chloride belongs to salt compound with chemical formula licl. As flame colorant lithium chloride is used to produce dark red flames. Molten licl is used for the preparation of lithium niobite graphene and carbon nanotubes.
 Source: fineartamerica.com
Source: fineartamerica.com
It went better than last year s demonstration i have 3 videos of those on youtube but not as g. A systemic resorption of lithium was shown in a study on 27 intensive care unit patients who were mechanically ventilated with lithium chloride coated heat and moisture exchangers for at least 5 days. Lithium may also be absorbed via the lungs. It is used as relative humidity standard in the calibration of hygrometers and itself can be used as a hygrometer. Even though original flame has orange ish color but the natural one has unstable color and less vibrant color.
 Source: youtube.com
Source: youtube.com
This process is used extensively in chemistry to determine what type and concentration of atoms a substance contains. Lithium emmited the least energy because according to the electromagnetic spectrum dark reds oranges and yellows have a high wavelength but low energy and lithium nitrate burned a dark red. Molten licl is used for the preparation of lithium niobite graphene and carbon nanotubes. Lithium i gives a pink flame copper ii gives a green flame and sodium i gives a yellow flame. It is used as relative humidity standard in the calibration of hygrometers and itself can be used as a hygrometer.
 Source: quizlet.com
Source: quizlet.com
Lithium chloride is used as a relative humidity standard in the calibration of hygrometers. The color is less intense than the strontium flame color. Lithium chloride imparts a red color to a flame. This process is used extensively in chemistry to determine what type and concentration of atoms a substance contains. As flame colorant lithium chloride is used to produce dark red flames.
 Source: cshakira1.blogspot.com
Source: cshakira1.blogspot.com
The color is less intense than the strontium flame color. Lithium nitrate and lithium chloride flame tests produce the same color because it is the lithium electrons that are raised to a higher energy level and then drop back down to their ground state. As flame colorant lithium chloride is used to produce dark red flames. Therefore to create red color we can use lithium chloride. The color is less intense than the strontium flame color.
 Source: reddit.com
Source: reddit.com
As flame colorant lithium chloride is used to produce dark red flames. This is the 08 09 school year s flame test demonstration. A systemic resorption of lithium was shown in a study on 27 intensive care unit patients who were mechanically ventilated with lithium chloride coated heat and moisture exchangers for at least 5 days. Molten licl is used for the preparation of lithium niobite graphene and carbon nanotubes. The color is less intense than the strontium flame color.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lithium chloride flame by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.