List transition metals
List Transition Metals. According to the study of chemical elements all elements are mainly classified into three main. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table. The 38 elements in groups 3 through 12 of the periodic table are called transition metals. Because scandium yttrium and lanthanum actually do not form compounds analogous to those of the other transition metals and because their chemistry is quite homologous to that of the lanthanoids they are excluded from the present discussion of the main transition metals.
 Chem4kids Com Elements Periodic Table Transition Metals From chem4kids.com
Chem4kids Com Elements Periodic Table Transition Metals From chem4kids.com
Transition metal ions commonly form octahedral complexes with small ligands e g. The 38 elements in groups 3 through 12 of the periodic table are called transition metals. Late transition metals are on the right side of the d block from group 8 to 11 and 12 if it is counted as transition metals. The interesting thing about transition metals is that their valence electrons or the electrons they use to combine with other elements are. The exception is mercury which is a liquid at room temperature. Square planar complexes are also formed e g.
According to the study of chemical elements all elements are mainly classified into three main.
Square planar complexes are also formed e g. These three main transition series are included in the set of 30 elements often called the d block transition metals. Transition metal ions commonly form octahedral complexes with small ligands e g. These metals are found in the earth s crust and ores of minerals. The interesting thing about transition metals is that their valence electrons or the electrons they use to combine with other elements are. According to the study of chemical elements all elements are mainly classified into three main.
 Source: m.youtube.com
Source: m.youtube.com
The exception is mercury which is a liquid at room temperature. The transition metals consist of the 40 elements located in columns 3 12 on the periodic table and the 28 elements comprising the lanthanide and actinide series. Square planar complexes are also formed e g. Transition metals like iron nickel and cobalt are known for producing a magnetic field. The general electronic configuration of the d block elements is n 1 d 1 10 ns 0 2.
 Source: sciencenotes.org
Source: sciencenotes.org
Because scandium yttrium and lanthanum actually do not form compounds analogous to those of the other transition metals and because their chemistry is quite homologous to that of the lanthanoids they are excluded from the present discussion of the main transition metals. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table. Ag nh3 2 used as tollen s reagent co nh3 6 2 cu h 2o 6 2 cocl4 2. Elements in the lanthanide and. As with all metals the transition elements are both ductile and malleable and conduct electricity and heat.
 Source: researchgate.net
Source: researchgate.net
The general electronic configuration of the d block elements is n 1 d 1 10 ns 0 2. Transition metal ions commonly form octahedral complexes with small ligands e g. The general electronic configuration of the d block elements is n 1 d 1 10 ns 0 2. Late transition metals are on the right side of the d block from group 8 to 11 and 12 if it is counted as transition metals. The 38 elements in groups 3 through 12 of the periodic table are called transition metals.
 Source: chubbyrevision-a2level.weebly.com
Source: chubbyrevision-a2level.weebly.com
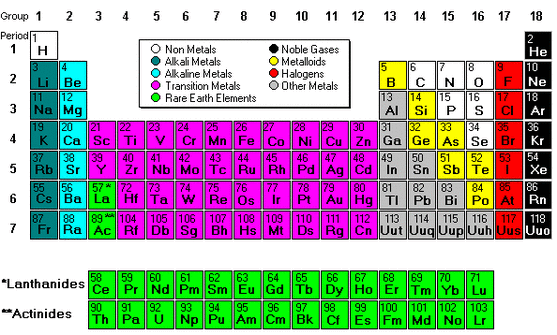
The transition metals consist of the 40 elements located in columns 3 12 on the periodic table and the 28 elements comprising the lanthanide and actinide series. The 38 elements in groups 3 through 12 of the periodic table are called transition metals. The transition metals as a group have high melting points. Here noble gas is the configuration of the. The transition metals consist of the 40 elements located in columns 3 12 on the periodic table and the 28 elements comprising the lanthanide and actinide series.
 Source: byjus.com
Source: byjus.com
The transition metals consist of the 40 elements located in columns 3 12 on the periodic table and the 28 elements comprising the lanthanide and actinide series. According to the study of chemical elements all elements are mainly classified into three main. The general electronic configuration of the d block elements is n 1 d 1 10 ns 0 2. Along with the transition metals you can also know more about the metals in other categories like rare earth elements and heavy metals. Late transition metals are on the right side of the d block from group 8 to 11 and 12 if it is counted as transition metals.
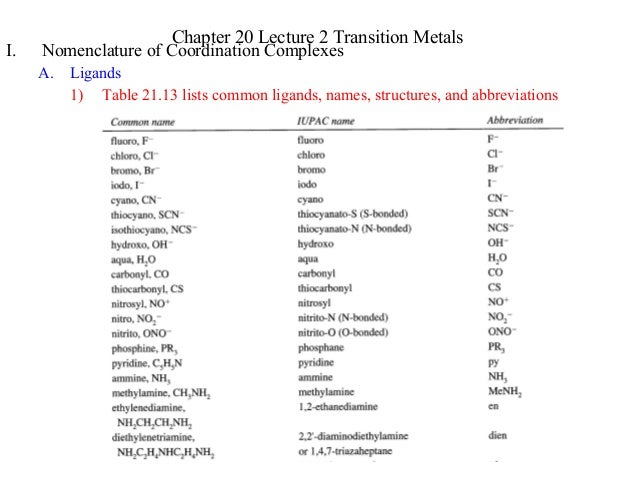 Source: slideshare.net
Source: slideshare.net
Transition metal ions commonly form octahedral complexes with small ligands e g. These metals are found in the earth s crust and ores of minerals. The exception is mercury which is a liquid at room temperature. Square planar complexes are also formed e g. These three main transition series are included in the set of 30 elements often called the d block transition metals.
 Source: study.com
Source: study.com
The transition metals consist of the 40 elements located in columns 3 12 on the periodic table and the 28 elements comprising the lanthanide and actinide series. Because scandium yttrium and lanthanum actually do not form compounds analogous to those of the other transition metals and because their chemistry is quite homologous to that of the lanthanoids they are excluded from the present discussion of the main transition metals. The interesting thing about transition metals is that their valence electrons or the electrons they use to combine with other elements are. As with all metals the transition elements are both ductile and malleable and conduct electricity and heat. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table.
 Source: chem4kids.com
Source: chem4kids.com
Cisplatin ag commonly forms linear complexes e g. The general electronic configuration of the d block elements is n 1 d 1 10 ns 0 2. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table. Transition metal ions commonly form tetrahedral complexes with larger ligands e g cl. Transition metals like iron nickel and cobalt are known for producing a magnetic field.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
By extension these elements also have high boiling points. The general electronic configuration of the d block elements is n 1 d 1 10 ns 0 2. Transition metal ions commonly form tetrahedral complexes with larger ligands e g cl. Late transition metals are on the right side of the d block from group 8 to 11 and 12 if it is counted as transition metals. By extension these elements also have high boiling points.
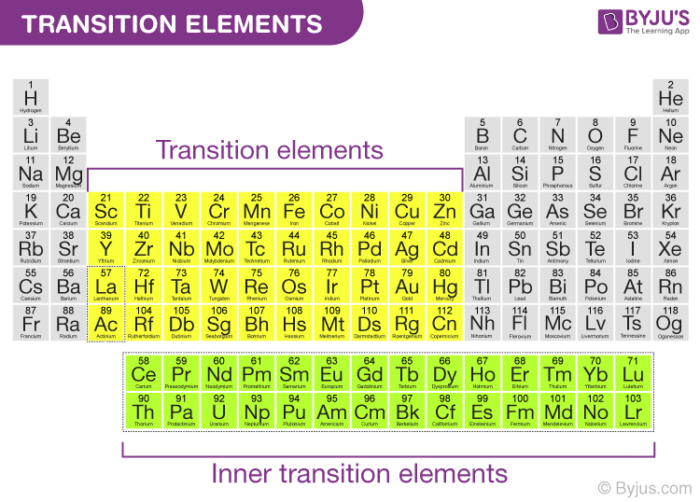 Source: byjus.com
Source: byjus.com
The 38 elements in groups 3 through 12 of the periodic table are called transition metals. Because scandium yttrium and lanthanum actually do not form compounds analogous to those of the other transition metals and because their chemistry is quite homologous to that of the lanthanoids they are excluded from the present discussion of the main transition metals. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table. The transition metals as a group have high melting points. Transition metals like iron nickel and cobalt are known for producing a magnetic field.
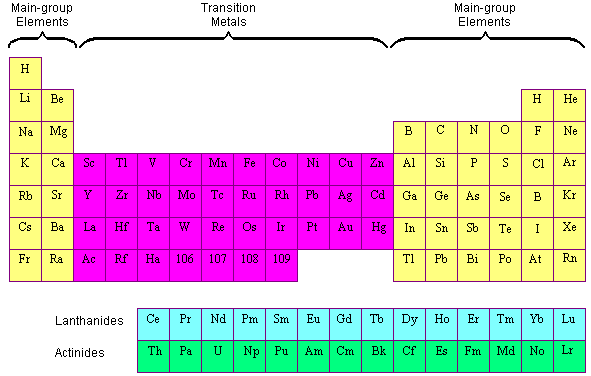 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
Late transition metals are on the right side of the d block from group 8 to 11 and 12 if it is counted as transition metals. Transition metal ions commonly form tetrahedral complexes with larger ligands e g cl. According to the study of chemical elements all elements are mainly classified into three main. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table. Because scandium yttrium and lanthanum actually do not form compounds analogous to those of the other transition metals and because their chemistry is quite homologous to that of the lanthanoids they are excluded from the present discussion of the main transition metals.
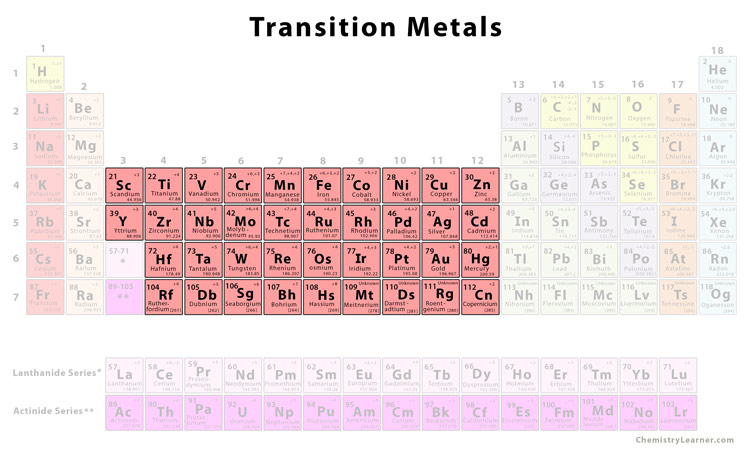 Source: chemistrylearner.com
Source: chemistrylearner.com
The exception is mercury which is a liquid at room temperature. These metals are found in the earth s crust and ores of minerals. The transition metals consist of the 40 elements located in columns 3 12 on the periodic table and the 28 elements comprising the lanthanide and actinide series. Square planar complexes are also formed e g. Cisplatin ag commonly forms linear complexes e g.
 Source: britannica.com
Source: britannica.com
Transition metals show characteristics like malleability ductility and are good conductor of electricity. Cisplatin ag commonly forms linear complexes e g. Late transition metals are on the right side of the d block from group 8 to 11 and 12 if it is counted as transition metals. Square planar complexes are also formed e g. Transition metals show characteristics like malleability ductility and are good conductor of electricity.
 Source: wikihow.com
Source: wikihow.com
The exception is mercury which is a liquid at room temperature. Here noble gas is the configuration of the. The 38 elements in groups 3 through 12 of the periodic table are called transition metals. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table. Transition metals like iron nickel and cobalt are known for producing a magnetic field.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Transition metals show characteristics like malleability ductility and are good conductor of electricity. Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table. The transition metals consist of the 40 elements located in columns 3 12 on the periodic table and the 28 elements comprising the lanthanide and actinide series. The general electronic configuration of the d block elements is n 1 d 1 10 ns 0 2. Transition metal ions commonly form octahedral complexes with small ligands e g.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title list transition metals by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.