Lemon battery hypothesis
Lemon Battery Hypothesis. Electricity is a electric power that is made by atoms and electrons. All from a lemon a penny and a nail. The insertion of two different metals used as anode and cathode into the lemon acting as the. The average of the phillips battery was 1 552 volts.
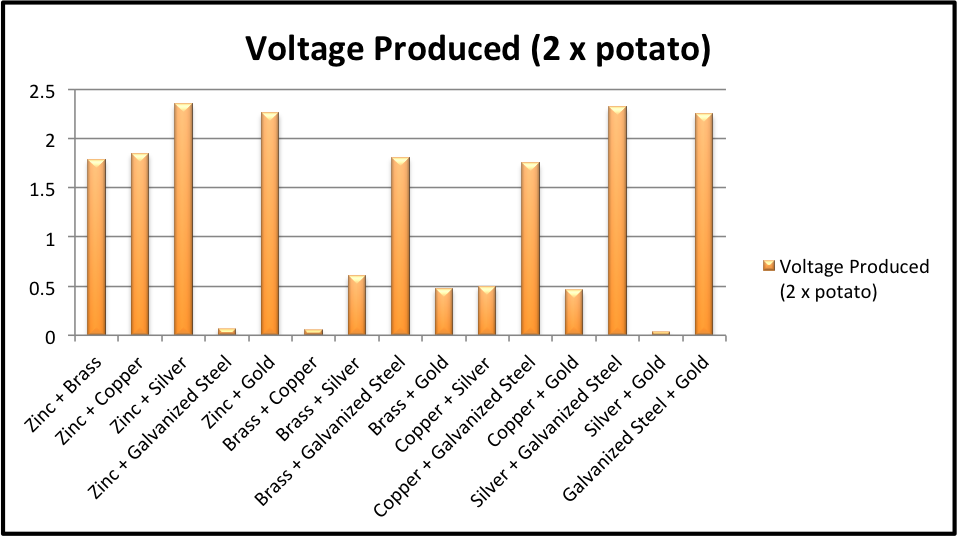 Experimental Record Chevron Lemons From chevronlemons.weebly.com
Experimental Record Chevron Lemons From chevronlemons.weebly.com
The lemon battery hypothesis states that a lemon is acidic enough to carry an electric charge and act as a battery. To demonstrate that a lemon can carry a. In a lemon battery the electricity comes from the chemical reaction between the copper zinc. My original hypothesis was correct. There are many variations of the lemon cell that use different fruits as electrolytes and metals other than zinc and copper as electrodes. It is possible to produce electric current using the acidic properties of lemon.
Always remember to be careful and safe around electricity.
All from a lemon a penny and a nail. The lemon battery illustrates the type of chemical reaction that occurs in batteries. The hypothesis of the lemon battery project is to demonstrate how a galvanic voltaic battery works. In this paragraph you learned what electricity is and the lemon is not the electricity. The lemon battery hypothesis states that a lemon is acidic enough to carry an electric charge and act as a battery. The higher the voltage the more power the battery has but higher voltage also means greater danger.
 Source: slideshare.net
Source: slideshare.net
The hypothesis of the lemon battery project is to demonstrate how a galvanic voltaic battery works. The higher the voltage the more power the battery has but higher voltage also means greater danger. The lemon battery is similar to the first electrical battery invented in 1800 by alessandro volta who used brine instead of lemon juice. In a lemon battery the electricity comes from the chemical reaction between the copper zinc. To test the lemon battery hypothesis students can follow these steps.
 Source: chevronlemons.weebly.com
Source: chevronlemons.weebly.com
The lemon battery hypothesis states that a lemon is acidic enough to carry an electric charge and act as a battery. To test the lemon battery hypothesis students can follow these steps. All from a lemon a penny and a nail. The lemon battery gave off a lot less voltage than the store bought batteries but still had the average of 214 volts. The insertion of two different metals used as anode and cathode into the lemon acting as the.
 Source: researchgate.net
Source: researchgate.net
The lemon battery is similar to the first electrical battery invented in 1800 by alessandro volta who used brine instead of lemon juice. In a lemon battery the electricity comes from the chemical reaction between the copper zinc. The zinc and copper are called the electrodes and the juice inside the lemon is called the electrolyte. My original hypothesis was correct. The lemon battery is similar to the first electrical battery invented in 1800 by alessandro volta who used brine instead of lemon juice.
 Source: slideplayer.com
Source: slideplayer.com
In a lemon battery the electricity comes from the chemical reaction between the copper zinc. In this paragraph you learned what electricity is and the lemon is not the electricity. Gather the right supplies. When you attach two electrodes to a lemon and touch your tongue to both of them at the same time it completes an electrical circuit making an electric current pass through the tongue giving it a tingling sensation or a metallic taste. All from a lemon a penny and a nail.
 Source: slideshare.net
Source: slideshare.net
The insertion of two different metals used as anode and cathode into the lemon acting as the. My original hypothesis was correct. The average of the phillips battery was 1 552 volts. The higher the voltage the more power the battery has but higher voltage also means greater danger. The zinc and copper are called the electrodes and the juice inside the lemon is called the electrolyte.
Source:
When you attach two electrodes to a lemon and touch your tongue to both of them at the same time it completes an electrical circuit making an electric current pass through the tongue giving it a tingling sensation or a metallic taste. Thankfully our lemon battery is very low voltage. The insertion of two different metals used as anode and cathode into the lemon acting as the. There are many variations of the lemon cell that use different fruits as electrolytes and metals other than zinc and copper as electrodes. In this paragraph you learned what electricity is and the lemon is not the electricity.
 Source: pinterest.com
Source: pinterest.com
The lemon battery gave off a lot less voltage than the store bought batteries but still had the average of 214 volts. Thankfully our lemon battery is very low voltage. In a lemon battery the electricity comes from the chemical reaction between the copper zinc. Gather the right supplies. The zinc and copper are called the electrodes and the juice inside the lemon is called the electrolyte.
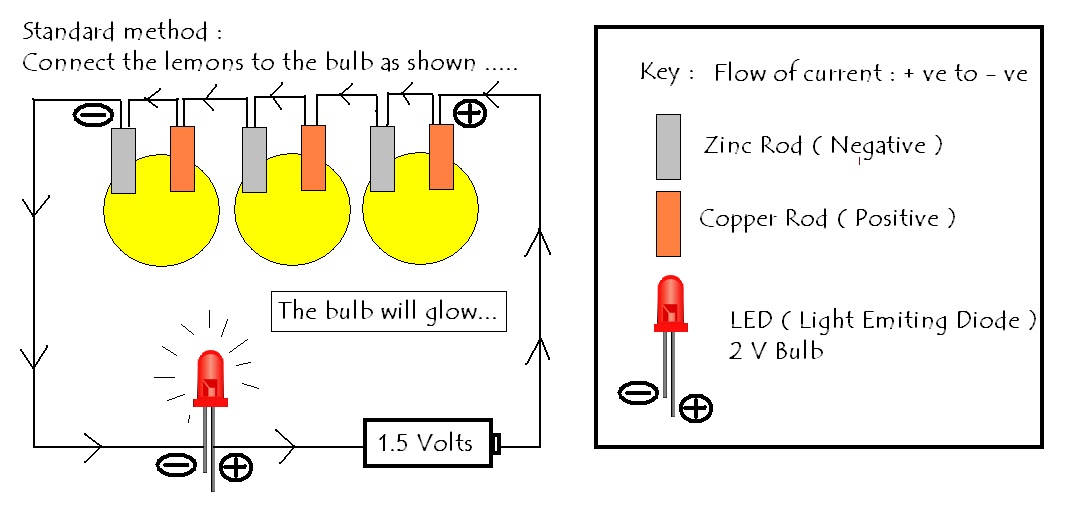 Source: vijaysaayi-experimentsandresults.blogspot.com
Source: vijaysaayi-experimentsandresults.blogspot.com
Electricity is a electric power that is made by atoms and electrons. The hypothesis of the lemon battery project is to demonstrate how a galvanic voltaic battery works. Volts or voltage is a measurement of the force moving the electrons through our lemon battery. The lemon battery hypothesis states that a lemon is acidic enough to carry an electric charge and act as a battery. All from a lemon a penny and a nail.
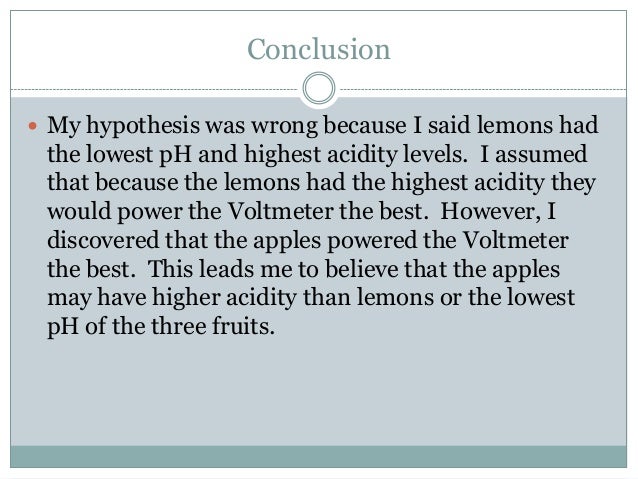 Source: pt.slideshare.net
Source: pt.slideshare.net
The lemon battery gave off a lot less voltage than the store bought batteries but still had the average of 214 volts. My original hypothesis was correct. To test the lemon battery hypothesis students can follow these steps. Electricity is a electric power that is made by atoms and electrons. The higher the voltage the more power the battery has but higher voltage also means greater danger.
 Source: pinterest.com
Source: pinterest.com
Thankfully our lemon battery is very low voltage. The zinc and copper are called the electrodes and the juice inside the lemon is called the electrolyte. The lemon battery hypothesis states that a lemon is acidic enough to carry an electric charge and act as a battery. Electricity is a electric power that is made by atoms and electrons. The higher the voltage the more power the battery has but higher voltage also means greater danger.
 Source: pt.slideshare.net
Source: pt.slideshare.net
The zinc and copper are called the electrodes and the juice inside the lemon is called the electrolyte. The average of the phillips battery was 1 552 volts. The lemon battery is similar to the first electrical battery invented in 1800 by alessandro volta who used brine instead of lemon juice. Electricity is a electric power that is made by atoms and electrons. To test the lemon battery hypothesis students can follow these steps.
 Source: pinterest.com
Source: pinterest.com
The hypothesis of the lemon battery project is to demonstrate how a galvanic voltaic battery works. When you attach two electrodes to a lemon and touch your tongue to both of them at the same time it completes an electrical circuit making an electric current pass through the tongue giving it a tingling sensation or a metallic taste. The lemon battery is similar to the first electrical battery invented in 1800 by alessandro volta who used brine instead of lemon juice. It is a electrical charge and it is the electrical current. The hypothesis of the lemon battery project is to demonstrate how a galvanic voltaic battery works.
 Source: slideshare.net
Source: slideshare.net
The lemon battery gave off a lot less voltage than the store bought batteries but still had the average of 214 volts. In a lemon battery the electricity comes from the chemical reaction between the copper zinc. The lemon battery gave off a lot less voltage than the store bought batteries but still had the average of 214 volts. The lemon battery hypothesis states that a lemon is acidic enough to carry an electric charge and act as a battery. Science fair projectsschool projectsprojects for kidsscience experiments kidsscience for kidsbeing a landlordelectric chargelemoneducation.
 Source: slideplayer.com
Source: slideplayer.com
The insertion of two different metals used as anode and cathode into the lemon acting as the. To demonstrate that a lemon can carry a. The higher the voltage the more power the battery has but higher voltage also means greater danger. It is possible to produce electric current using the acidic properties of lemon. There are many variations of the lemon cell that use different fruits as electrolytes and metals other than zinc and copper as electrodes.
 Source: youtube.com
Source: youtube.com
Always remember to be careful and safe around electricity. The lemon battery illustrates the type of chemical reaction that occurs in batteries. My original hypothesis was correct. There are many variations of the lemon cell that use different fruits as electrolytes and metals other than zinc and copper as electrodes. All from a lemon a penny and a nail.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lemon battery hypothesis by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






