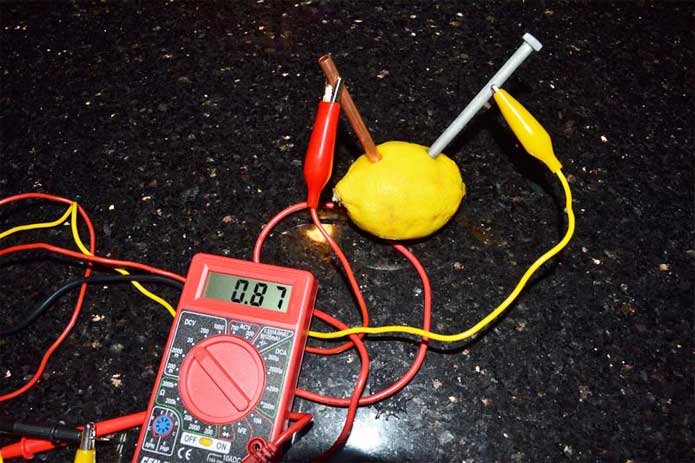
Lemon and potato battery
Lemon And Potato Battery. The lemon juice is acidic and works as a electrolyte. An hypothesis is a prediction of what you think might happen in a experiment or test. A fruit or vegetable doesn t exactly produce electricity it just provides a conductive liquid that completes the circuit. According to naked scientists contributor dave ansell their lemon battery would have required 5 000 hours to charge their battery and he predicted it would have most likely died within a mere 30 minutes.
 Lemon Battery Wikipedia From en.wikipedia.org
Lemon Battery Wikipedia From en.wikipedia.org
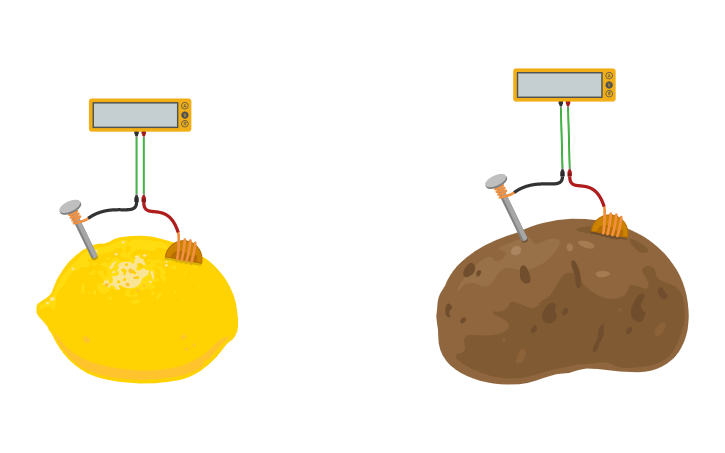
We predict the lemons will produce more voltage then potatoes. This is because i think the amount of liquid and acid affects the amount of electricity conducted and lemons. Batteries with different electrode shapes or surface areas might have different internal resistances. Battery cells made with different electrode materials like copper nickel or zinc might produce different voltages. A fruit or vegetable doesn t exactly produce electricity it just provides a conductive liquid that completes the circuit. In this experiment the zinc nail was the anode and the copper wire was the cathode the electrolyte was the lemon potato juice.
By david s and luqman a from 7 2 thanks for watching observation we saw that the lemon and the potato did not work out the way it was suppose to work we thought it would have least a little.
However the potato battery was definitely a lot more work. Both were able to light up our led light bulbs so in that sense they are both successful. Batteries with different electrode shapes or surface areas might have different internal resistances. Battery cells made with different electrode materials like copper nickel or zinc might produce different voltages. So now we have made both a lemon battery and a potato battery which one is better. This is how our lemon battery will look for the experiment.
 Source: sciencewithkids.com
Source: sciencewithkids.com
However the potato battery was definitely a lot more work. According to naked scientists contributor dave ansell their lemon battery would have required 5 000 hours to charge their battery and he predicted it would have most likely died within a mere 30 minutes. An hypothesis is a prediction of what you think might happen in a experiment or test. We predict the lemons will produce more voltage then potatoes. So if you are looking for a quicker experiment the lemon battery is faster and easier.
 Source: sciencewithkids.com
Source: sciencewithkids.com
An hypothesis is a prediction of what you think might happen in a experiment or test. Batteries with different electrode shapes or surface areas might have different internal resistances. In this experiment the zinc nail was the anode and the copper wire was the cathode the electrolyte was the lemon potato juice. Battery cells made with different electrode materials like copper nickel or zinc might produce different voltages. The lemon itself composes the transfer of electrons to and from the electrolyte.
 Source: elliottelectronicsupply.com
Source: elliottelectronicsupply.com
Connect the two potatoes with one alligator clip lead. Finishing this step the two potatoes should be attached to each other and the clock. The lemon juice is acidic and works as a electrolyte. Battery cells made with different electrode materials like copper nickel or zinc might produce different voltages. This is how our lemon battery will look for the experiment.
 Source: ameliascience7.weebly.com
Source: ameliascience7.weebly.com
This is how our lemon battery will look for the experiment. The lemon itself composes the transfer of electrons to and from the electrolyte. The lemon potato battery produces electricity by converting chemical energy to electrical energy. An hypothesis is a prediction of what you think might happen in a experiment or test. This will complete the circuit for your battery.
 Source: varvarag.wordpress.com
Source: varvarag.wordpress.com
We predict the lemons will produce more voltage then potatoes. Potatoes volts 1 0 84v 2 1 63v 3 2 48v 4 3 18v yes conclusion. We predict the lemons will produce more voltage then potatoes. By david s and luqman a from 7 2 thanks for watching observation we saw that the lemon and the potato did not work out the way it was suppose to work we thought it would have least a little. The lemon potato battery produces electricity by converting chemical energy to electrical energy.
 Source: pinterest.com
Source: pinterest.com
Batteries with different electrode shapes or surface areas might have different internal resistances. We predict the lemons will produce more voltage then potatoes. Both were able to light up our led light bulbs so in that sense they are both successful. This is how our lemon battery will look for the experiment. This is because i think the amount of liquid and acid affects the amount of electricity conducted and lemons.
 Source: ameliascience7.weebly.com
Source: ameliascience7.weebly.com
Potatoes volts 1 0 84v 2 1 63v 3 2 48v 4 3 18v yes conclusion. In this experiment the zinc nail was the anode and the copper wire was the cathode the electrolyte was the lemon potato juice. This will complete the circuit for your battery. A battery cell made with a potato might provide a different amount of current than a battery cell made with a lemon or an onion. The lemon potato battery produces electricity by converting chemical energy to electrical energy.
 Source: indiamart.com
Source: indiamart.com
Batteries with different electrode shapes or surface areas might have different internal resistances. Attach one clip to the galvanized nail in the first potato and the other clip to the copper coin in the second potato. Connect the two potatoes with one alligator clip lead. In this experiment the zinc nail was the anode and the copper wire was the cathode the electrolyte was the lemon potato juice. A battery cell made with a potato might provide a different amount of current than a battery cell made with a lemon or an onion.
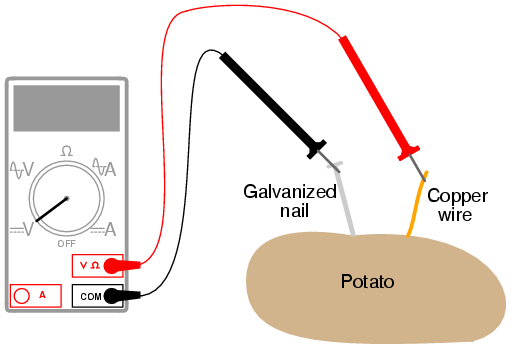 Source: bristolwatch.com
Source: bristolwatch.com
In this experiment the zinc nail was the anode and the copper wire was the cathode the electrolyte was the lemon potato juice. However the potato battery was definitely a lot more work. This is because i think the amount of liquid and acid affects the amount of electricity conducted and lemons. According to naked scientists contributor dave ansell their lemon battery would have required 5 000 hours to charge their battery and he predicted it would have most likely died within a mere 30 minutes. This will complete the circuit for your battery.
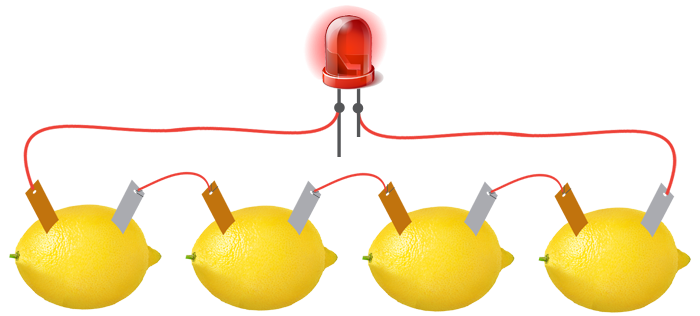 Source: kit4curious.com
Source: kit4curious.com
So if you are looking for a quicker experiment the lemon battery is faster and easier. The lemon itself composes the transfer of electrons to and from the electrolyte. According to naked scientists contributor dave ansell their lemon battery would have required 5 000 hours to charge their battery and he predicted it would have most likely died within a mere 30 minutes. In this experiment the zinc nail was the anode and the copper wire was the cathode the electrolyte was the lemon potato juice. However the potato battery was definitely a lot more work.
 Source: ameliascience7.weebly.com
Source: ameliascience7.weebly.com
So if you are looking for a quicker experiment the lemon battery is faster and easier. Both were able to light up our led light bulbs so in that sense they are both successful. A battery cell made with a potato might provide a different amount of current than a battery cell made with a lemon or an onion. This is how our lemon battery will look for the experiment. This is because i think the amount of liquid and acid affects the amount of electricity conducted and lemons.
 Source: en.wikipedia.org
Source: en.wikipedia.org
So if you are looking for a quicker experiment the lemon battery is faster and easier. This is how our lemon battery will look for the experiment. So now we have made both a lemon battery and a potato battery which one is better. Both were able to light up our led light bulbs so in that sense they are both successful. This will complete the circuit for your battery.
 Source: m.youtube.com
Source: m.youtube.com
We predict the lemons will produce more voltage then potatoes. According to naked scientists contributor dave ansell their lemon battery would have required 5 000 hours to charge their battery and he predicted it would have most likely died within a mere 30 minutes. We predict the lemons will produce more voltage then potatoes. This is how our lemon battery will look for the experiment. By david s and luqman a from 7 2 thanks for watching observation we saw that the lemon and the potato did not work out the way it was suppose to work we thought it would have least a little.
 Source: sciencewithkids.com
Source: sciencewithkids.com
This is how our lemon battery will look for the experiment. By david s and luqman a from 7 2 thanks for watching observation we saw that the lemon and the potato did not work out the way it was suppose to work we thought it would have least a little. We predict the lemons will produce more voltage then potatoes. This is because i think the amount of liquid and acid affects the amount of electricity conducted and lemons. The lemon juice is acidic and works as a electrolyte.
 Source: tinkercad.com
Source: tinkercad.com
However the potato battery was definitely a lot more work. A battery cell made with a potato might provide a different amount of current than a battery cell made with a lemon or an onion. The lemon juice is acidic and works as a electrolyte. A fruit or vegetable doesn t exactly produce electricity it just provides a conductive liquid that completes the circuit. This is how our lemon battery will look for the experiment.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lemon and potato battery by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.