Is salt a good conductor of electricity
Is Salt A Good Conductor Of Electricity. Yes salt water is a good conductor of electricity na and cl ions will help in conduction of electricity. Distilled water is a a good conductor of electricity b poor conductor. When sodium chloride dissolves in water the water separates the sodium and chlorine ions. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current.
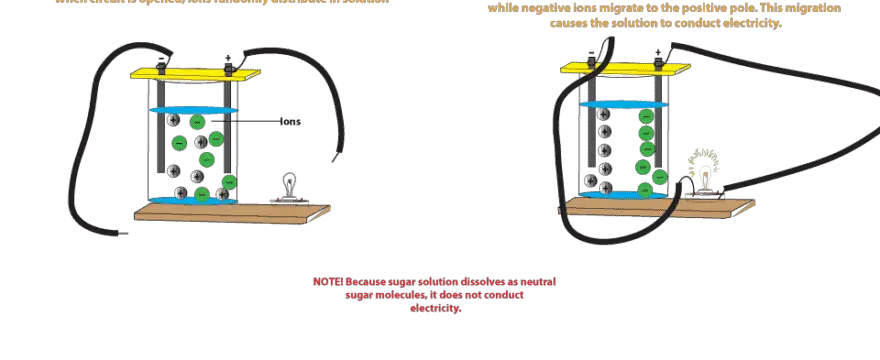 Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T From masterconceptsinchemistry.com
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T From masterconceptsinchemistry.com
Substances such as salts acids and hydroxides that also are electrolytes can conduct electric current. In the solid state however the ions are trapped in a lattice by electrostatic forces. This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy. Saltwater is a good conductor of electricity because it is an electrolyte solution. Why does a salt not conduct electricity in its solid state. In order to conduct electricity charged particles must be available and these charged particles must have the possibily to move around freely.
Saltwater is a good conductor of electricity because it is an electrolyte solution.
In the solid state however the ions are trapped in a lattice by electrostatic forces. An ion is an atom that has an electrical charge because it has either gained or lost an electron also meaning it has a positive charge and a negative charge. Salts consist of ions. Adding common salt to distilled water makes it a good conductor b poor conductor c both d none 2. When sodium chloride dissolves in water the water separates the sodium and chlorine ions. These ions being charged particles can conduct electricity.
 Source: edwardparkerj01h.jkub.com
Source: edwardparkerj01h.jkub.com
Why does a salt not conduct electricity in its solid state. Saltwater is a mixture that consists of water and sodium chloride. Salts consist of ions. This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy. In fact sea water is a much better conductor of electricity than normal water.
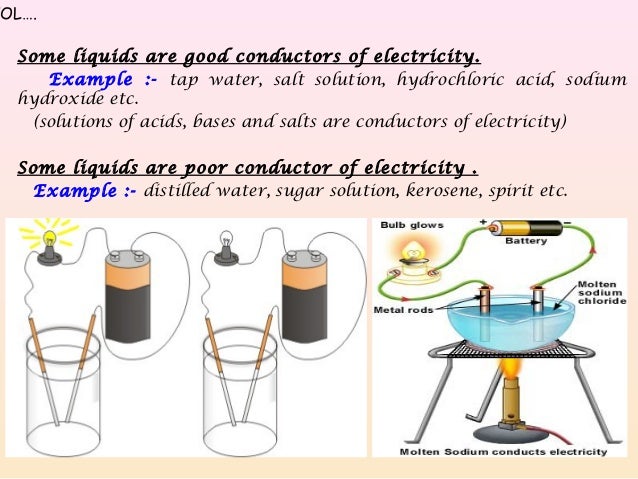 Source: slideshare.net
Source: slideshare.net
Distilled water is a a good conductor of electricity b poor conductor. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. Distilled water is a a good conductor of electricity b poor conductor. An electrolyte is a a metal b solution c liquid that conducts current d none of these 3. In the solid state however the ions are trapped in a lattice by electrostatic forces.
 Source: teachoo.com
Source: teachoo.com
Salt molecules are made of sodium ions and chlorine ions. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. Yes salt water is a good conductor of electricity na and cl ions will help in conduction of electricity. Why does a salt not conduct electricity in its solid state. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl.
Source: homesciencetools.com
In order to conduct electricity charged particles must be available and these charged particles must have the possibily to move around freely. Sea water contains common salt sodium chloride n a c l and other compounds which when dissolved in water dissociate into ions in the case of n a c l it produces n a and c l ions. Electricity is a steady flow of electrons or electrically charged particles through a substance. Salt molecules are made of sodium ions and chlorine ions. Saltwater is a good conductor of electricity because it is an electrolyte solution.
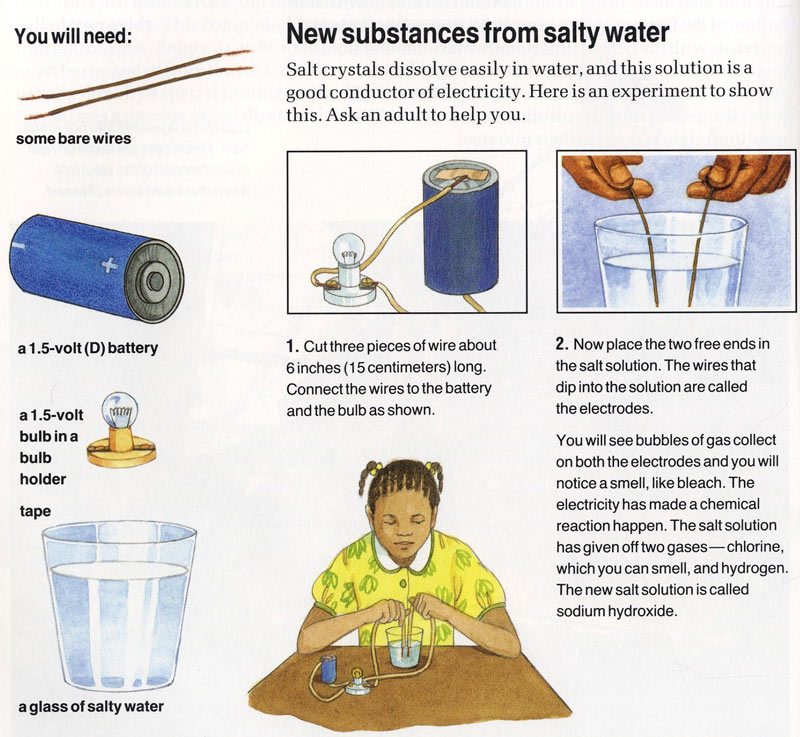 Source: ency123.com
Source: ency123.com
In order to conduct electricity charged particles must be available and these charged particles must have the possibily to move around freely. Sea water contains common salt sodium chloride n a c l and other compounds which when dissolved in water dissociate into ions in the case of n a c l it produces n a and c l ions. Substances such as salts acids and hydroxides that also are electrolytes can conduct electric current. Saltwater is a mixture that consists of water and sodium chloride. In the solid state however the ions are trapped in a lattice by electrostatic forces.
 Source: youtube.com
Source: youtube.com
Electricity is a steady flow of electrons or electrically charged particles through a substance. Substances such as salts acids and hydroxides that also are electrolytes can conduct electric current. Sea water contains common salt sodium chloride n a c l and other compounds which when dissolved in water dissociate into ions in the case of n a c l it produces n a and c l ions. Adding common salt to distilled water makes it a good conductor b poor conductor c both d none 2. An ion is an atom that has an electrical charge because it has either gained or lost an electron also meaning it has a positive charge and a negative charge.
 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
Substances such as salts acids and hydroxides that also are electrolytes can conduct electric current. An ion is an atom that has an electrical charge because it has either gained or lost an electron also meaning it has a positive charge and a negative charge. Why does a salt not conduct electricity in its solid state. In fact sea water is a much better conductor of electricity than normal water. Distilled water is a a good conductor of electricity b poor conductor.
 Source: slideplayer.com
Source: slideplayer.com
Salts consist of ions. Salt molecules are made of sodium ions and chlorine ions. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current. An electrolyte is a a metal b solution c liquid that conducts current d none of these 3. In order to conduct electricity charged particles must be available and these charged particles must have the possibily to move around freely.
 Source: teachengineering.org
Source: teachengineering.org
In other conductors such as salt water the current is moved by molecules called ions. These ions being charged particles can conduct electricity. In fact sea water is a much better conductor of electricity than normal water. Saltwater is a mixture that consists of water and sodium chloride. Distilled water is a a good conductor of electricity b poor conductor.
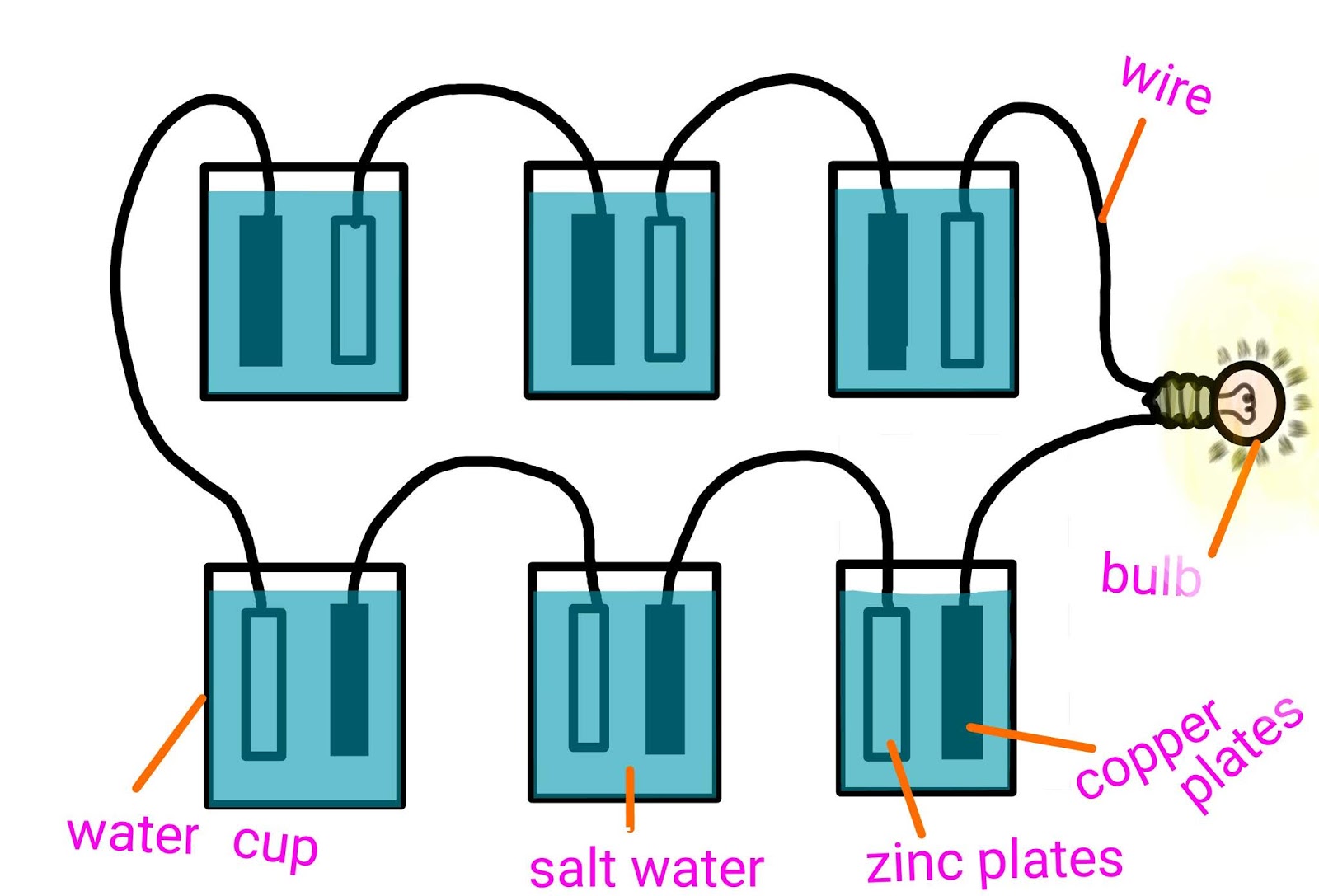 Source: vikasrajcreations.blogspot.com
Source: vikasrajcreations.blogspot.com
Saltwater is a mixture that consists of water and sodium chloride. In fact sea water is a much better conductor of electricity than normal water. Why does a salt not conduct electricity in its solid state. Distilled water is a a good conductor of electricity b poor conductor. In some conductors such as copper the electrons themselves are able to flow through the substance carrying the current.
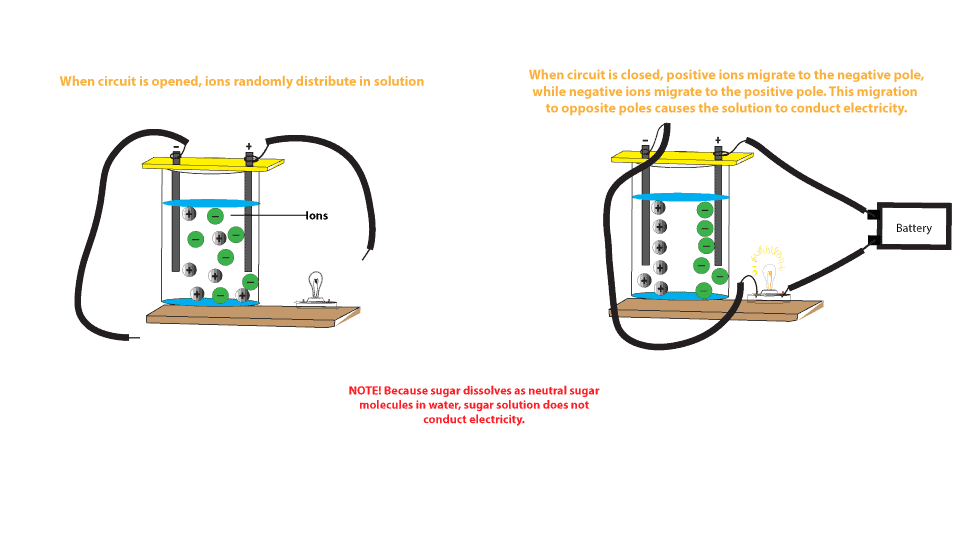 Source: masterconceptsinchemistry.com
Source: masterconceptsinchemistry.com
Distilled water is a a good conductor of electricity b poor conductor. Salt solution such as sodium chloride nacl conducts an electric current because it has ions in it that have the freedom to move about in solution. These ions being charged particles can conduct electricity. Saltwater is a mixture that consists of water and sodium chloride. Yes salt water is a good conductor of electricity na and cl ions will help in conduction of electricity.
 Source: rookieparenting.com
Source: rookieparenting.com
An electrolyte is a a metal b solution c liquid that conducts current d none of these 3. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl. In other conductors such as salt water the current is moved by molecules called ions. This is because salt water is a good conductor of electricity which makes ocean water a resource for renewable energy. Salts consist of ions.
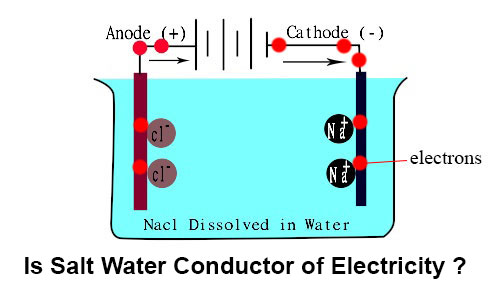 Source: ekunji.com
Source: ekunji.com
Saltwater is a mixture that consists of water and sodium chloride. Saltwater is a good conductor of electricity because it is an electrolyte solution. Saltwater is a mixture that consists of water and sodium chloride. Salt molecules are made of sodium ions and chlorine ions. These ions are produced when sodium chloride dissolves in pure water to produce sodium na and chloride ions cl.
 Source: youtube.com
Source: youtube.com
In order to conduct electricity charged particles must be available and these charged particles must have the possibily to move around freely. These ions being charged particles can conduct electricity. Saltwater is a good conductor of electricity because it is an electrolyte solution. Salt molecules are made of sodium ions and chlorine ions. Electricity is a steady flow of electrons or electrically charged particles through a substance.
 Source: examfear.com
Source: examfear.com
Yes salt water is a good conductor of electricity na and cl ions will help in conduction of electricity. These ions being charged particles can conduct electricity. In the solid state however the ions are trapped in a lattice by electrostatic forces. Salt molecules are made of sodium ions and chlorine ions. In order to conduct electricity charged particles must be available and these charged particles must have the possibily to move around freely.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is salt a good conductor of electricity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.