Is magnesium a alkali metal
Is Magnesium A Alkali Metal. The alkali metals are so reactive that they are generally found in nature combined with other elements. The elements have very similar properties. Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra. A similar effect occurs for magnesium fluoride consistent with the diagonal relationship between lithium and magnesium.

These are often used as reducing agents producing hydrides. The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. All are shiny fairly soft although harder than the alkali metals and most are white or silvery colored. For instance lithium frames a steady nitride a property normal among all the soluble earth metals magnesium s group however exceptional among the alkali metals. Further among their particular groups just lithium and magnesium structure organometallic compounds with critical covalent character for example lime and mgme2. Li na k rb cs b lithium is the only alkali metal to form a nitride directly.
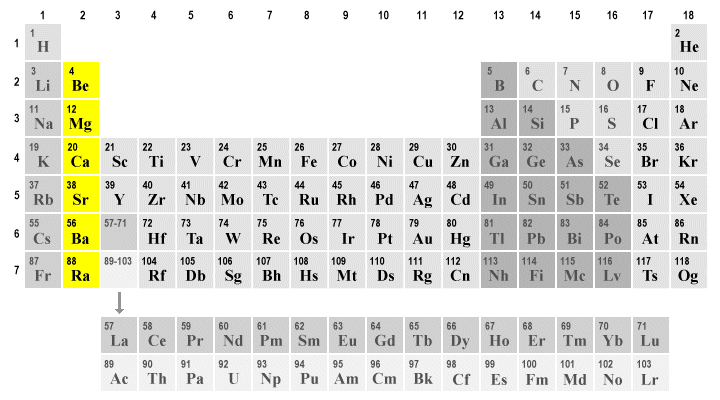
Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra.
Beryllium magnesium calcium strontium barium and radium. Because the outer electron structure in all of these elements is similar they all have somewhat similar chemical and physical properties. The smaller the size of the ion the. The elements have very similar properties. These are often used as reducing agents producing hydrides. The high lattice enthalpy of lithium fluoride is due to the small sizes of the li and f ions causing the electrostatic interactions between them to be strong.

These are often used as reducing agents producing hydrides. C eo for m 2 aq 2e m s where m ca sr or ba is nearly constant. The smaller the size of the ion the. The high lattice enthalpy of lithium fluoride is due to the small sizes of the li and f ions causing the electrostatic interactions between them to be strong. Alkaline earth metals alkaline earth material is any element that you can find in the second column of the periodic table for example.
 Source: thoughtco.com
Source: thoughtco.com
Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra. For instance lithium frames a steady nitride a property normal among all the soluble earth metals magnesium s group however exceptional among the alkali metals. The smaller the size of the ion the. A the alkali metal ions are highly hydrated. The mobilities of the alkali metal ions in aqueous solution are.
 Source: technologyuk.net
Source: technologyuk.net
The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. A similar effect occurs for magnesium fluoride consistent with the diagonal relationship between lithium and magnesium. The alkali metals are so reactive that they are generally found in nature combined with other elements. For instance lithium frames a steady nitride a property normal among all the soluble earth metals magnesium s group however exceptional among the alkali metals. Simple minerals such as halite sodium chloride nacl sylvite potassium chloride kcl and carnallite a potassium magnesium chloride kcl mgcl 2 6h 2 o are soluble in water and therefore are easily extracted and purified.
 Source: researchgate.net
Source: researchgate.net
C eo for m 2 aq 2e m s where m ca sr or ba is nearly constant. Li na k rb cs b lithium is the only alkali metal to form a nitride directly. The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. For instance lithium frames a steady nitride a property normal among all the soluble earth metals magnesium s group however exceptional among the alkali metals. The smaller the size of the ion the.
 Source: owenandkadin.weebly.com
Source: owenandkadin.weebly.com
C eo for m 2 aq 2e m s where m ca sr or ba is nearly constant. The high lattice enthalpy of lithium fluoride is due to the small sizes of the li and f ions causing the electrostatic interactions between them to be strong. The smaller the size of the ion the. The alkali metals are so reactive that they are generally found in nature combined with other elements. The alkali metals also react similarly with hydrogen to form ionic alkali metal hydrides where the hydride anion acts as a pseudohalide.
 Source: scienceclarified.com
Source: scienceclarified.com
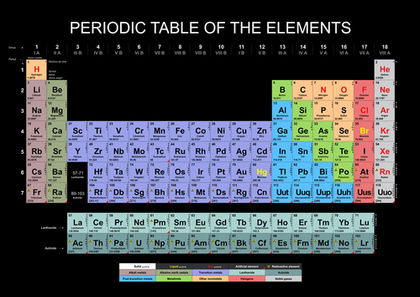
They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. A the alkali metal ions are highly hydrated. The alkali metals also react similarly with hydrogen to form ionic alkali metal hydrides where the hydride anion acts as a pseudohalide. Structurally they together with helium have in common an outer s. The mobilities of the alkali metal ions in aqueous solution are.
 Source: researchgate.net
Source: researchgate.net
Structurally they together with helium have in common an outer s. The mobilities of the alkali metal ions in aqueous solution are. Because the outer electron structure in all of these elements is similar they all have somewhat similar chemical and physical properties. Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra. The elements have very similar properties.
 Source: marketcap.com.au
Source: marketcap.com.au
They are all shiny silvery white somewhat reactive metals at standard temperature and pressure. The smaller the size of the ion the. Alkaline earth metals are metals that have two valence electrons in their outermost shell. The alkali metals also react similarly with hydrogen to form ionic alkali metal hydrides where the hydride anion acts as a pseudohalide. The alkali metals are so reactive that they are generally found in nature combined with other elements.
 Source: chegg.com
Source: chegg.com
A the alkali metal ions are highly hydrated. There are six alkaline earth metals including beryllium magnesium calcium strontium barium and radium. Beryllium magnesium calcium strontium barium and radium. The high lattice enthalpy of lithium fluoride is due to the small sizes of the li and f ions causing the electrostatic interactions between them to be strong. The alkali metals also react similarly with hydrogen to form ionic alkali metal hydrides where the hydride anion acts as a pseudohalide.
 Source: periodictablegroups.wordpress.com
Source: periodictablegroups.wordpress.com
Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra. Beryllium magnesium calcium strontium barium and radium. A the alkali metal ions are highly hydrated. For instance lithium frames a steady nitride a property normal among all the soluble earth metals magnesium s group however exceptional among the alkali metals. The mobilities of the alkali metal ions in aqueous solution are.
 Source: azchemistry.com
Source: azchemistry.com
These are often used as reducing agents producing hydrides. Simple minerals such as halite sodium chloride nacl sylvite potassium chloride kcl and carnallite a potassium magnesium chloride kcl mgcl 2 6h 2 o are soluble in water and therefore are easily extracted and purified. Alkaline earth metals alkaline earth material is any element that you can find in the second column of the periodic table for example. They become stable by gaining the electron configuration of noble gasses through the donation of their outermost electrons. The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra.

Simple minerals such as halite sodium chloride nacl sylvite potassium chloride kcl and carnallite a potassium magnesium chloride kcl mgcl 2 6h 2 o are soluble in water and therefore are easily extracted and purified. The alkaline earth metals are six chemical elements in group 2 of the periodic table they are beryllium be magnesium mg calcium ca strontium sr barium ba and radium ra. The mobilities of the alkali metal ions in aqueous solution are. For instance lithium frames a steady nitride a property normal among all the soluble earth metals magnesium s group however exceptional among the alkali metals. C eo for m 2 aq 2e m s where m ca sr or ba is nearly constant.
 Source: toppr.com
Source: toppr.com
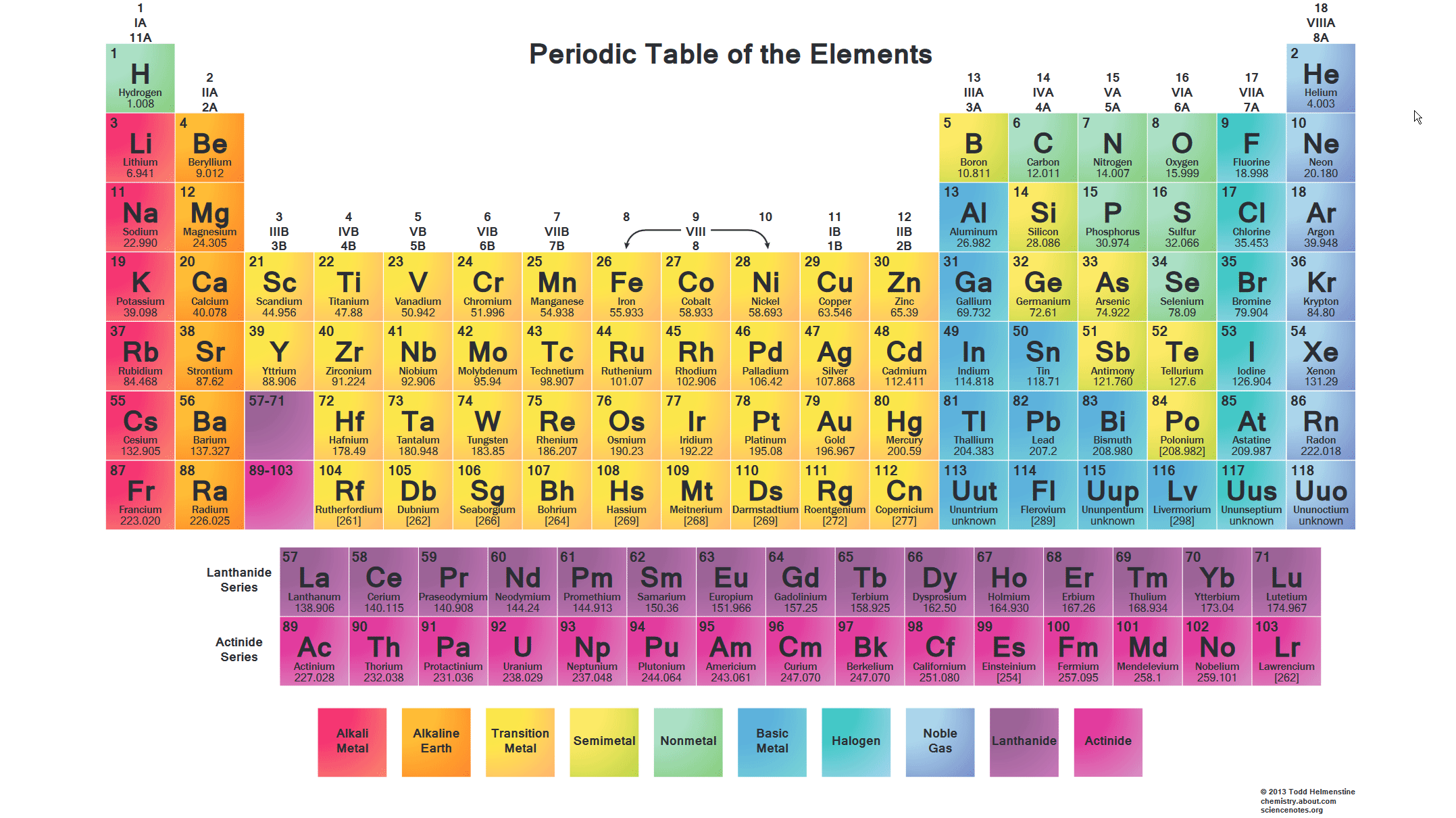
The alkali metals are so reactive that they are generally found in nature combined with other elements. The second column on the periodic table of the chemical elements is collectively called the alkaline earth metal group. Further among their particular groups just lithium and magnesium structure organometallic compounds with critical covalent character for example lime and mgme2. They become stable by gaining the electron configuration of noble gasses through the donation of their outermost electrons. For instance lithium frames a steady nitride a property normal among all the soluble earth metals magnesium s group however exceptional among the alkali metals.
 Source: britannica.com
Source: britannica.com
Simple minerals such as halite sodium chloride nacl sylvite potassium chloride kcl and carnallite a potassium magnesium chloride kcl mgcl 2 6h 2 o are soluble in water and therefore are easily extracted and purified. Simple minerals such as halite sodium chloride nacl sylvite potassium chloride kcl and carnallite a potassium magnesium chloride kcl mgcl 2 6h 2 o are soluble in water and therefore are easily extracted and purified. The mobilities of the alkali metal ions in aqueous solution are. Alkaline earth metals are metals that have two valence electrons in their outermost shell. All are shiny fairly soft although harder than the alkali metals and most are white or silvery colored.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
All are shiny fairly soft although harder than the alkali metals and most are white or silvery colored. A the alkali metal ions are highly hydrated. Li na k rb cs b lithium is the only alkali metal to form a nitride directly. A similar effect occurs for magnesium fluoride consistent with the diagonal relationship between lithium and magnesium. The high lattice enthalpy of lithium fluoride is due to the small sizes of the li and f ions causing the electrostatic interactions between them to be strong.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is magnesium a alkali metal by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.