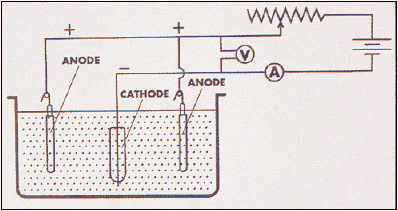
Is iron an alkali metal
Is Iron An Alkali Metal. No it is a transition metal. Francium is the most metallic element both in group and the periodic table. Alkali metals are highly conductive soft metals which have a high lustre that oxidizes quickly. This was first noted by humphry davy in 1809 and rediscovered by w.
 Alkali Metal Wikipedia From en.wikipedia.org
Alkali Metal Wikipedia From en.wikipedia.org
The alkali metals dissolve slowly in liquid ammonia forming ammoniacal solutions of solvated metal cation m and solvated electron e which react to form hydrogen gas and the alkali metal amide mnh 2 where m represents an alkali metal. Atomic radius moving from the top to the bottom of the column in group 2 will increase the number of. This was first noted by humphry davy in 1809 and rediscovered by w. Cesium is the most volatile of the alkali metals with a boiling point of 671 c 1 240 f. Francium is the most metallic element both in group and the periodic table. Alkali metals have been studied since 1807 when sir humphry davy explored the electrical properties of potassium and sodium.
Alkali metal is the name given to the first group of elements in the periodic table.
The alkali metals dissolve slowly in liquid ammonia forming ammoniacal solutions of solvated metal cation m and solvated electron e which react to form hydrogen gas and the alkali metal amide mnh 2 where m represents an alkali metal. These metals also have unusual physical properties for metals. Atomic radius moving from the top to the bottom of the column in group 2 will increase the number of. Less commonly metals including iron copper zinc aluminum beryllium cobalt manganese and arsenic may be considered heavy metals. Cesium is the most volatile of the alkali metals with a boiling point of 671 c 1 240 f. This is why they are called alkali metals.
 Source: en.wikipedia.org
Source: en.wikipedia.org
They have low melting points increasing up the group from 28 c for cs to 180 c for li. Like the other elements in group 1 hydrogen h has one electron in its outermost shell but it is not classed as an alkali metal since it is not a metal but a gas at room temperature periodic table of the elements. They form basic oxides. This was first noted by humphry davy in 1809 and rediscovered by w. Atomic radius moving from the top to the bottom of the column in group 2 will increase the number of.
 Source: en.wikipedia.org
Source: en.wikipedia.org
They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr. Cesium is the most volatile of the alkali metals with a boiling point of 671 c 1 240 f. Alkali metals are highly conductive soft metals which have a high lustre that oxidizes quickly. No it is a transition metal. They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr.
 Source: ar.pinterest.com
Source: ar.pinterest.com
Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra. Alkali metals particularly sodium are important in commercial use and chemical synthesis. Like the other elements in group 1 hydrogen h has one electron in its outermost shell but it is not classed as an alkali metal since it is not a metal but a gas at room temperature periodic table of the elements. This was first noted by humphry davy in 1809 and rediscovered by w. Properties of alkali metals.
 Source: quora.com
Source: quora.com
They form basic oxides. The alkali metals dissolve slowly in liquid ammonia forming ammoniacal solutions of solvated metal cation m and solvated electron e which react to form hydrogen gas and the alkali metal amide mnh 2 where m represents an alkali metal. Alkali metals have been studied since 1807 when sir humphry davy explored the electrical properties of potassium and sodium. They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr. Is iron an alkali metal.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Less commonly metals including iron copper zinc aluminum beryllium cobalt manganese and arsenic may be considered heavy metals. Less commonly metals including iron copper zinc aluminum beryllium cobalt manganese and arsenic may be considered heavy metals. Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra. Properties of alkali metals. Alkali metals are highly conductive soft metals which have a high lustre that oxidizes quickly.
 Source: slideplayer.com
Source: slideplayer.com
They form basic oxides. Alkali metals are highly conductive soft metals which have a high lustre that oxidizes quickly. They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr. Alkali metals particularly sodium are important in commercial use and chemical synthesis. Alkaline earth metals alkaline earth material is any element that you can find in the second column of the periodic table for example.
 Source: study.com
Source: study.com
Is iron an alkali metal. No it is a transition metal. Is iron an alkali metal. List of heavy metals if you go by the definition of a heavy metal as a metallic element with a density greater than 5 then the list of heavy metals is. Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra.
 Source: docbrown.info
Source: docbrown.info
They have low melting points increasing up the group from 28 c for cs to 180 c for li. The alkali metals dissolve slowly in liquid ammonia forming ammoniacal solutions of solvated metal cation m and solvated electron e which react to form hydrogen gas and the alkali metal amide mnh 2 where m represents an alkali metal. This is why they are called alkali metals. No it is a transition metal. Atomic radius moving from the top to the bottom of the column in group 2 will increase the number of.
 Source: periodictablegroups.wordpress.com
Source: periodictablegroups.wordpress.com
Alkali metals have been studied since 1807 when sir humphry davy explored the electrical properties of potassium and sodium. Like the other elements in group 1 hydrogen h has one electron in its outermost shell but it is not classed as an alkali metal since it is not a metal but a gas at room temperature periodic table of the elements. This was first noted by humphry davy in 1809 and rediscovered by w. Francium is the most metallic element both in group and the periodic table. Atomic radius moving from the top to the bottom of the column in group 2 will increase the number of.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The alkali metals dissolve slowly in liquid ammonia forming ammoniacal solutions of solvated metal cation m and solvated electron e which react to form hydrogen gas and the alkali metal amide mnh 2 where m represents an alkali metal. List of heavy metals if you go by the definition of a heavy metal as a metallic element with a density greater than 5 then the list of heavy metals is. They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr. They form basic oxides. No it is a transition metal.
 Source: twitter.com
Source: twitter.com
Atomic radius moving from the top to the bottom of the column in group 2 will increase the number of. This is why they are called alkali metals. Francium is the most metallic element both in group and the periodic table. They have low melting points increasing up the group from 28 c for cs to 180 c for li. No it is a transition metal.
 Source: britannica.com
Source: britannica.com
Atomic radius moving from the top to the bottom of the column in group 2 will increase the number of. All of the alkali merals are metals as they lose electron to form cations. They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr. Alkaline earth metals alkaline earth material is any element that you can find in the second column of the periodic table for example. They have 1 valence electron and thus valency 1.
 Source: link.springer.com
Source: link.springer.com
Like the other elements in group 1 hydrogen h has one electron in its outermost shell but it is not classed as an alkali metal since it is not a metal but a gas at room temperature periodic table of the elements. Alkali metals particularly sodium are important in commercial use and chemical synthesis. These metals also have unusual physical properties for metals. The boiling points of the alkali metals decrease in regular fashion as the atomic numbers increase with the highest 1 317 c 2 403 f being that of lithium. This is why they are called alkali metals.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Alkali metals particularly sodium are important in commercial use and chemical synthesis. Less commonly metals including iron copper zinc aluminum beryllium cobalt manganese and arsenic may be considered heavy metals. Alkali metal is the name given to the first group of elements in the periodic table. The boiling points of the alkali metals decrease in regular fashion as the atomic numbers increase with the highest 1 317 c 2 403 f being that of lithium. They are lithium li sodium na potassium k rubidium rb cesium cs and francium fr.
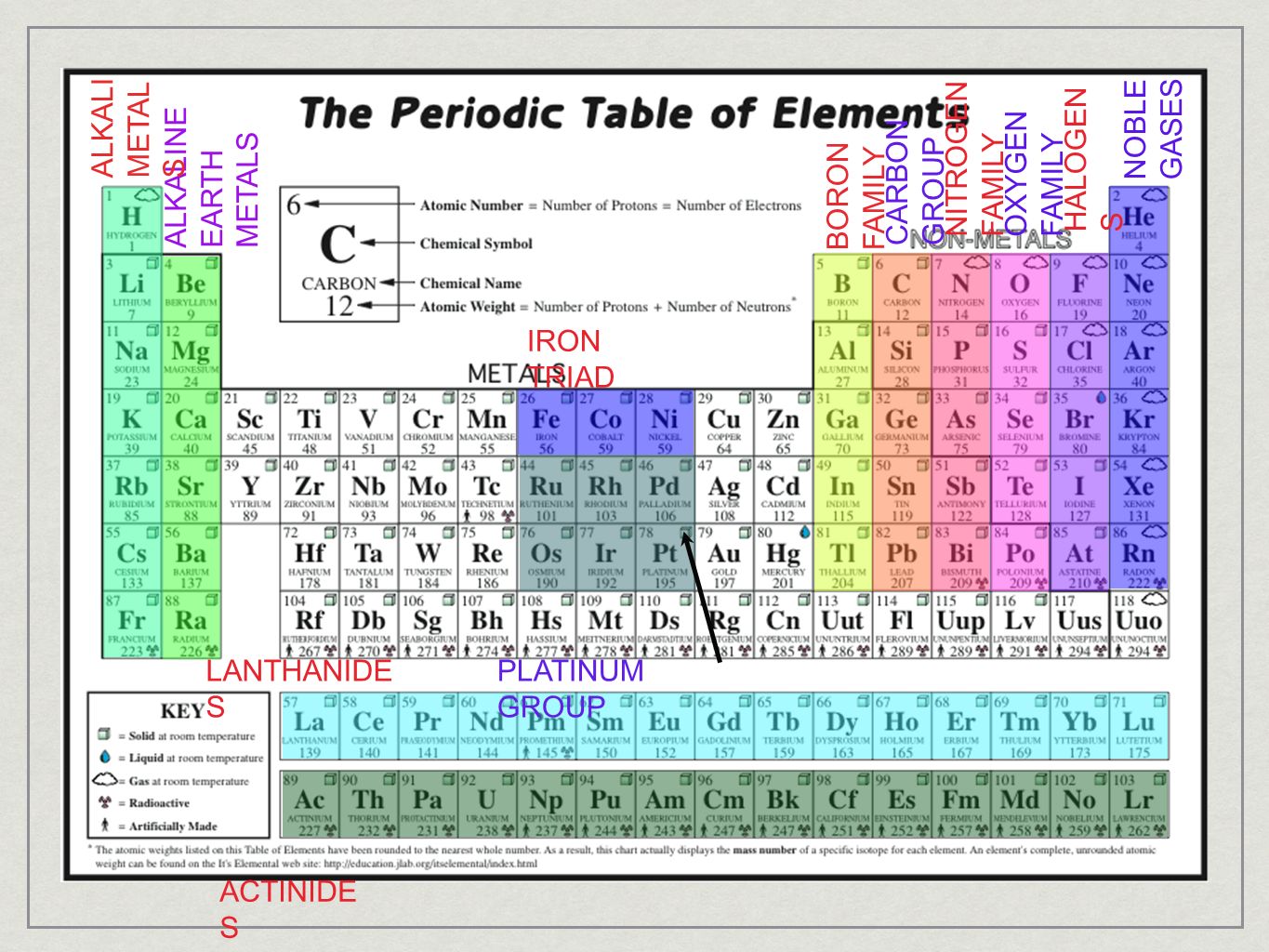 Source: slideplayer.com
Source: slideplayer.com
Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra. Atomic radius moving from the top to the bottom of the column in group 2 will increase the number of. Beryllium be magnesium mg calcium ca strontium sr barium ba radium ra. Less commonly metals including iron copper zinc aluminum beryllium cobalt manganese and arsenic may be considered heavy metals. No it is a transition metal.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is iron an alkali metal by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.