Is iron a pure substance or mixture
Is Iron A Pure Substance Or Mixture. In chemistry a pure substance consists of only one type of atom molecule or compound. A mixture can be separated into its. No chemical process occurs and the material can be identified as a mixture by the fact that the sulfur and the iron can be separated by a mechanical process such as using a magnet to attract the iron away from the sulfur. If so you have a pure substance.
 Difference Between Pure Substance And Mixture Guidance Corner From guidancecorner.com
Difference Between Pure Substance And Mixture Guidance Corner From guidancecorner.com
Iron is a pure substance. The measure of whether a substance is pure or not is known as purity a pure element or compound contains only one substance with no other substances mixed in. A pure substance is any form of matter that has a uniform composition and cannot be separated by physical methods such as filtration. An alloy is a mixture of chemical elements which forms an impure substance admixture that retains the characteristics of a metal an alloy is distinct from an impure metal in that with an alloy the added elements are well controlled to produce desirable properties while impure metals such as wrought iron are less controlled but are often considered useful. One example of a mixture is oil in water. A pure substance is made up of only one substance and can t be separated into any other substance.
A pure substance is any form of matter that has a uniform composition and cannot be separated by physical methods such as filtration.
Pure substances cannot be separated into other substances. Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas. Click to see full answer. No chemical process occurs and the material can be identified as a mixture by the fact that the sulfur and the iron can be separated by a mechanical process such as using a magnet to attract the iron away from the sulfur. One example of a mixture is oil in water. Iron is a pure substance.

Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas. Those substances which are made with a single type of atoms or contain only one type of particles having fixed and constant structures are called pure substances. Mixtures contain two or more substances and can be physically separated. No chemical process occurs and the material can be identified as a mixture by the fact that the sulfur and the iron can be separated by a mechanical process such as using a magnet to attract the iron away from the sulfur. Iron is a pure substance.

One example of a pure substance is iron. Those substances which are made with a single type of atoms or contain only one type of particles having fixed and constant structures are called pure substances. Pure substances cannot be separated into other substances. For example pure iron would only contain iron atoms whereas pure sugar would contain molecules of sucrose. If so you have a pure substance.
 Source: chegg.com
Source: chegg.com
Mixtures contain two or more substances and can be physically separated. One example of a pure substance is iron. Aluminum iron silver and gold are some common examples of pure substances. No chemical process occurs and the material can be identified as a mixture by the fact that the sulfur and the iron can be separated by a mechanical process such as using a magnet to attract the iron away from the sulfur. Iron is a pure substance.
 Source: 3aapeh3ke.changeip.org
Source: 3aapeh3ke.changeip.org
A mixture can be separated into its. In chemistry a pure substance consists of only one type of atom molecule or compound. A mixture can be separated into its. If not you have a mixture. Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas.
 Source: youtube.com
Source: youtube.com
Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas. If so you have a pure substance. Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas. A pure substance is made up of only one substance and can t be separated into any other substance. Pure substances cannot be separated into other substances.
 Source: sciencemaster.co.uk
Source: sciencemaster.co.uk
For example pure iron would only contain iron atoms whereas pure sugar would contain molecules of sucrose. No chemical process occurs and the material can be identified as a mixture by the fact that the sulfur and the iron can be separated by a mechanical process such as using a magnet to attract the iron away from the sulfur. Mixtures contain two or more substances and can be physically separated. For example pure iron would only contain iron atoms whereas pure sugar would contain molecules of sucrose. Aluminum iron silver and gold are some common examples of pure substances.
 Source: 3aapeh3ke.changeip.org
Source: 3aapeh3ke.changeip.org
Aluminum iron silver and gold are some common examples of pure substances. Pure substances cannot be separated into other substances. An alloy is a mixture of chemical elements which forms an impure substance admixture that retains the characteristics of a metal an alloy is distinct from an impure metal in that with an alloy the added elements are well controlled to produce desirable properties while impure metals such as wrought iron are less controlled but are often considered useful. If so you have a pure substance. Click to see full answer.
 Source: slideplayer.com
Source: slideplayer.com
One example of a pure substance is iron. Aluminum iron silver and gold are some common examples of pure substances. One example of a mixture is oil in water. Iron is a pure substance. If not you have a mixture.
 Source: thoughtco.com
Source: thoughtco.com
Aluminum iron silver and gold are some common examples of pure substances. One example of a pure substance is iron. A pure substance is any form of matter that has a uniform composition and cannot be separated by physical methods such as filtration. Aluminum iron silver and gold are some common examples of pure substances. Iron is a pure substance.
 Source: slideplayer.com
Source: slideplayer.com
An alloy is a mixture of chemical elements which forms an impure substance admixture that retains the characteristics of a metal an alloy is distinct from an impure metal in that with an alloy the added elements are well controlled to produce desirable properties while impure metals such as wrought iron are less controlled but are often considered useful. An alloy is a mixture of chemical elements which forms an impure substance admixture that retains the characteristics of a metal an alloy is distinct from an impure metal in that with an alloy the added elements are well controlled to produce desirable properties while impure metals such as wrought iron are less controlled but are often considered useful. If so you have a pure substance. A pure substance is any form of matter that has a uniform composition and cannot be separated by physical methods such as filtration. A pure substance is made up of only one substance and can t be separated into any other substance.
 Source: slideserve.com
Source: slideserve.com
The measure of whether a substance is pure or not is known as purity a pure element or compound contains only one substance with no other substances mixed in. One example of a mixture is oil in water. Click to see full answer. If not you have a mixture. No chemical process occurs and the material can be identified as a mixture by the fact that the sulfur and the iron can be separated by a mechanical process such as using a magnet to attract the iron away from the sulfur.
 Source: thoughtco.com
Source: thoughtco.com
In chemistry a pure substance consists of only one type of atom molecule or compound. Iron is a pure substance. Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas. In chemistry a pure substance consists of only one type of atom molecule or compound. No chemical process occurs and the material can be identified as a mixture by the fact that the sulfur and the iron can be separated by a mechanical process such as using a magnet to attract the iron away from the sulfur.
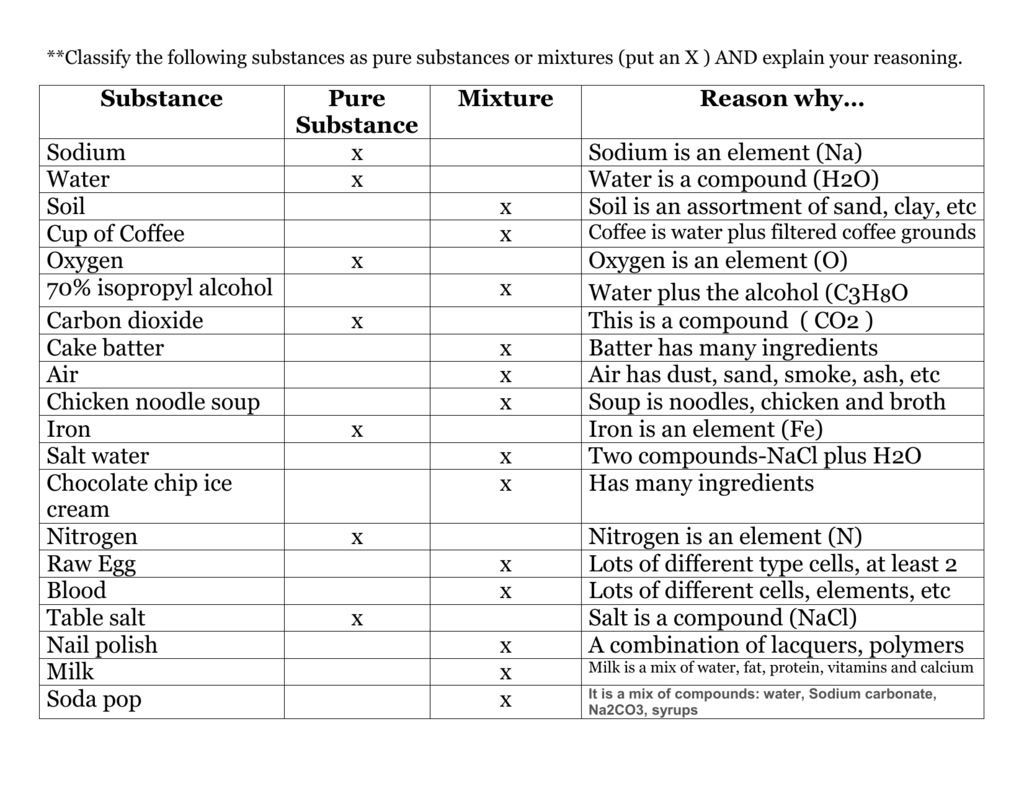 Source: studylib.net
Source: studylib.net
Iron is a pure substance. A pure substance is any form of matter that has a uniform composition and cannot be separated by physical methods such as filtration. One example of a pure substance is iron. A pure substance is made up of only one substance and can t be separated into any other substance. Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas.
 Source: slideserve.com
Source: slideserve.com
Pure substances cannot be separated into other substances. Click to see full answer. Aluminum iron silver and gold are some common examples of pure substances. One example of a pure substance is iron. Those substances which are made with a single type of atoms or contain only one type of particles having fixed and constant structures are called pure substances.
 Source: guidancecorner.com
Source: guidancecorner.com
A pure substance is any form of matter that has a uniform composition and cannot be separated by physical methods such as filtration. If so you have a pure substance. Click to see full answer. An alloy is a mixture of chemical elements which forms an impure substance admixture that retains the characteristics of a metal an alloy is distinct from an impure metal in that with an alloy the added elements are well controlled to produce desirable properties while impure metals such as wrought iron are less controlled but are often considered useful. Some examples are carbon iron water sugar salt nitrogen gas and oxygen gas.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title is iron a pure substance or mixture by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





