Is copper corrosion resistant
Is Copper Corrosion Resistant. The potential range of aluminum alloy lies between 0 6 and 0 8 v vs the saturated calomel electrode. Bronze is a mixture of copper and tin along with small amounts of other elements and is naturally much more resistant to corrosion than copper. Brass is an alloy of copper zinc and other elements which also resists corrosion. High velocity and turbulent flow conditions can remove these films and permit local rapid corrosion.
 Zirconium Deoxidation And Corrosion Resistance Properties For Copper Belmont Metals From belmontmetals.com
Zirconium Deoxidation And Corrosion Resistance Properties For Copper Belmont Metals From belmontmetals.com
Bronze is a mixture of copper and tin along with small amounts of other elements and is naturally much more resistant to corrosion than copper. Copper has been shown to significantly improve corrosion resistance of stainless steels particularly in seawater and sulphuric acid containing environments. Brass is a copper alloy containing a large amount of zinc. Brass is an alloy of copper zinc and other elements which also resists corrosion. The good resistance of copper pipe alloys to corrosion by seawater depends partly upon the inherent cathodic nobility of the metal but it also depends on copper s ability to form a protective passive film. High velocity and turbulent flow conditions can remove these films and permit local rapid corrosion.
So even if the two metals are almost equal in corrosion resistance there are many varieties of stainless steel some of which are exrtemely resistant to corrosion treating them as equals is not good for corrosion resistance.
Copper is resistant to most organic chemicals and can operate indefinitely in most industrial environments. Copper has been shown to significantly improve corrosion resistance of stainless steels particularly in seawater and sulphuric acid containing environments. The potential range of aluminum alloy lies between 0 6 and 0 8 v vs the saturated calomel electrode. If aluminum is connected to copper corrosion potential 0 1 v a significant difference of potential 0 5 v would exist and aluminum being anodic to copper would corrode. Brass is an alloy of copper zinc and other elements which also resists corrosion. Bronze is a copper alloy that includes a large amount of tin and smaller amounts of other alloying elements.
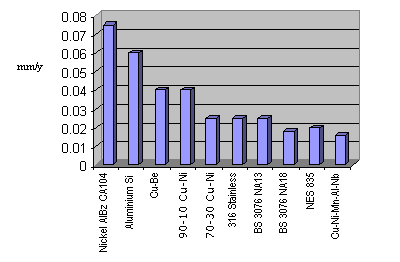 Source: copper.org
Source: copper.org
So even if the two metals are almost equal in corrosion resistance there are many varieties of stainless steel some of which are exrtemely resistant to corrosion treating them as equals is not good for corrosion resistance. If aluminum is connected to copper corrosion potential 0 1 v a significant difference of potential 0 5 v would exist and aluminum being anodic to copper would corrode. The good resistance of copper pipe alloys to corrosion by seawater depends partly upon the inherent cathodic nobility of the metal but it also depends on copper s ability to form a protective passive film. For copper corrosion is not an issue. High velocity and turbulent flow conditions can remove these films and permit local rapid corrosion.
 Source: slideshare.net
Source: slideshare.net
Coupling of copper with aluminum or steel can lead to severe galvanic corrosion. The potential range of aluminum alloy lies between 0 6 and 0 8 v vs the saturated calomel electrode. Copper brass and bronze are also corrosion resistant metals. However any corrosion oxides chlorides and sulfides that do form on copper are somewhat conductive. So even if the two metals are almost equal in corrosion resistance there are many varieties of stainless steel some of which are exrtemely resistant to corrosion treating them as equals is not good for corrosion resistance.
 Source: researchgate.net
Source: researchgate.net
Coupling of copper with aluminum or steel can lead to severe galvanic corrosion. Zaki ahmad in principles of corrosion engineering and corrosion control 2006. Copper generally resists corrosion from moisture humidity industrial pollution and other atmospheric influences. The good resistance of copper pipe alloys to corrosion by seawater depends partly upon the inherent cathodic nobility of the metal but it also depends on copper s ability to form a protective passive film. Copper and copper alloys are widely used in many environments and applications because of their excellent corrosion resistance which is coupled with combinations of other desirable properties such as superior electrical and thermal conductivity ease of fabricating and joining wide range of attainable mechanical properties and resistance to biofouling.
 Source: wiley.com
Source: wiley.com
The potential range of aluminum alloy lies between 0 6 and 0 8 v vs the saturated calomel electrode. A green patina may be formed after long exposure to the atmosphere but this is a function of the protective surface film and does not indicate a harmful attack. And salt water it s normal is especially tough on stainless steel. Copper and copper alloys are widely used in many environments and applications because of their excellent corrosion resistance which is coupled with combinations of other desirable properties such as superior electrical and thermal conductivity ease of fabricating and joining wide range of attainable mechanical properties and resistance to biofouling. The corrosion resistance of stainless steels is due to the highly stable passive film that forms on its surface when exposed to an oxygen containing environment.
 Source: belmontmetals.com
Source: belmontmetals.com
Copper is resistant to most organic chemicals and can operate indefinitely in most industrial environments. Brass is a copper alloy containing a large amount of zinc. Copper has been shown to significantly improve corrosion resistance of stainless steels particularly in seawater and sulphuric acid containing environments. Copper and copper alloys are widely used in many environments and applications because of their excellent corrosion resistance which is coupled with combinations of other desirable properties such as superior electrical and thermal conductivity ease of fabricating and joining wide range of attainable mechanical properties and resistance to biofouling. Copper generally resists corrosion from moisture humidity industrial pollution and other atmospheric influences.
 Source: beetleplastics.com
Source: beetleplastics.com
For copper corrosion is not an issue. Coupling of copper with aluminum or steel can lead to severe galvanic corrosion. Bronze is a copper alloy that includes a large amount of tin and smaller amounts of other alloying elements. Brass is an alloy of copper zinc and other elements which also resists corrosion. Today copper nickels have an established reputation for handling seawater in a wide range of conditions and applications.
 Source: totalmateria.com
Source: totalmateria.com
Copper generally resists corrosion from moisture humidity industrial pollution and other atmospheric influences. Copper has been shown to significantly improve corrosion resistance of stainless steels particularly in seawater and sulphuric acid containing environments. Zaki ahmad in principles of corrosion engineering and corrosion control 2006. If aluminum is connected to copper corrosion potential 0 1 v a significant difference of potential 0 5 v would exist and aluminum being anodic to copper would corrode. And salt water it s normal is especially tough on stainless steel.
 Source: enggweb.com
Source: enggweb.com
Brass is an alloy of copper zinc and other elements which also resists corrosion. Brass is a copper alloy containing a large amount of zinc. A green patina may be formed after long exposure to the atmosphere but this is a function of the protective surface film and does not indicate a harmful attack. However any corrosion oxides chlorides and sulfides that do form on copper are somewhat conductive. If aluminum is connected to copper corrosion potential 0 1 v a significant difference of potential 0 5 v would exist and aluminum being anodic to copper would corrode.
 Source: steeljrv.com
Source: steeljrv.com
High velocity and turbulent flow conditions can remove these films and permit local rapid corrosion. Bronze is a mixture of copper and tin along with small amounts of other elements and is naturally much more resistant to corrosion than copper. Copper is resistant to most organic chemicals and can operate indefinitely in most industrial environments. Copper and copper alloys are widely used in many environments and applications because of their excellent corrosion resistance which is coupled with combinations of other desirable properties such as superior electrical and thermal conductivity ease of fabricating and joining wide range of attainable mechanical properties and resistance to biofouling. And salt water it s normal is especially tough on stainless steel.
 Source: nickelinstitute.org
Source: nickelinstitute.org
However any corrosion oxides chlorides and sulfides that do form on copper are somewhat conductive. Zaki ahmad in principles of corrosion engineering and corrosion control 2006. A green patina may be formed after long exposure to the atmosphere but this is a function of the protective surface film and does not indicate a harmful attack. High velocity and turbulent flow conditions can remove these films and permit local rapid corrosion. Bronze is a mixture of copper and tin along with small amounts of other elements and is naturally much more resistant to corrosion than copper.
 Source: scielo.br
Source: scielo.br
Coupling of copper with aluminum or steel can lead to severe galvanic corrosion. The corrosion resistance of stainless steels is due to the highly stable passive film that forms on its surface when exposed to an oxygen containing environment. Cyanides are also very corrosive to copper pipes. Copper generally resists corrosion from moisture humidity industrial pollution and other atmospheric influences. High velocity and turbulent flow conditions can remove these films and permit local rapid corrosion.
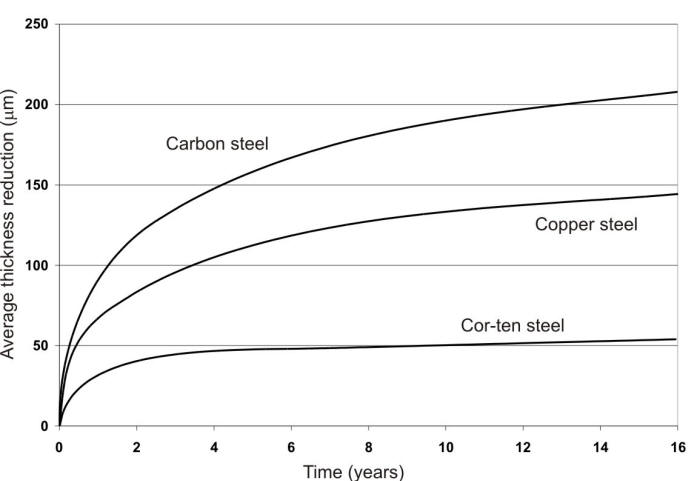 Source: corrosion-doctors.org
Source: corrosion-doctors.org
Copper has been shown to significantly improve corrosion resistance of stainless steels particularly in seawater and sulphuric acid containing environments. The potential range of aluminum alloy lies between 0 6 and 0 8 v vs the saturated calomel electrode. Coupling of copper with aluminum or steel can lead to severe galvanic corrosion. So even if the two metals are almost equal in corrosion resistance there are many varieties of stainless steel some of which are exrtemely resistant to corrosion treating them as equals is not good for corrosion resistance. Corrosion resistance copper nickel sheathing as corrosion protection in the splash zone 90 10 and 70 30 copper nickels were originally developed for naval condensers and piping.
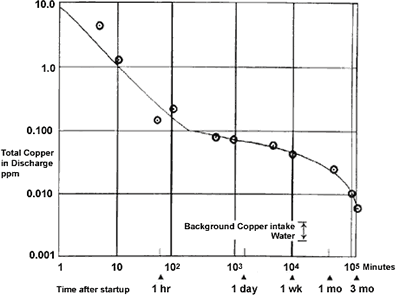 Source: copper.org
Source: copper.org
Corrosion resistance copper nickel sheathing as corrosion protection in the splash zone 90 10 and 70 30 copper nickels were originally developed for naval condensers and piping. High velocity and turbulent flow conditions can remove these films and permit local rapid corrosion. Copper and copper alloys are widely used in many environments and applications because of their excellent corrosion resistance which is coupled with combinations of other desirable properties such as superior electrical and thermal conductivity ease of fabricating and joining wide range of attainable mechanical properties and resistance to biofouling. Zaki ahmad in principles of corrosion engineering and corrosion control 2006. Copper brass and bronze are also corrosion resistant metals.
 Source: ngk-insulators.com
Source: ngk-insulators.com
If aluminum is connected to copper corrosion potential 0 1 v a significant difference of potential 0 5 v would exist and aluminum being anodic to copper would corrode. Corrosion is the unwanted breakdown and weakening of a material due to chemical reactions. The potential range of aluminum alloy lies between 0 6 and 0 8 v vs the saturated calomel electrode. Copper brass and bronze are also corrosion resistant metals. However any corrosion oxides chlorides and sulfides that do form on copper are somewhat conductive.
 Source: copper.org
Source: copper.org
For copper corrosion is not an issue. Copper and copper alloys are widely used in many environments and applications because of their excellent corrosion resistance which is coupled with combinations of other desirable properties such as superior electrical and thermal conductivity ease of fabricating and joining wide range of attainable mechanical properties and resistance to biofouling. Today copper nickels have an established reputation for handling seawater in a wide range of conditions and applications. Cyanides are also very corrosive to copper pipes. Corrosion resistance copper nickel sheathing as corrosion protection in the splash zone 90 10 and 70 30 copper nickels were originally developed for naval condensers and piping.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is copper corrosion resistant by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.