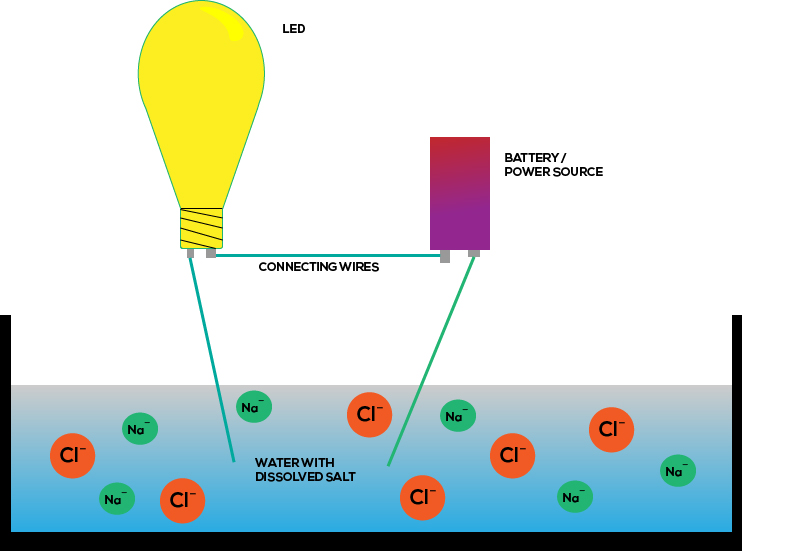
Is carbon a good conductor of electricity
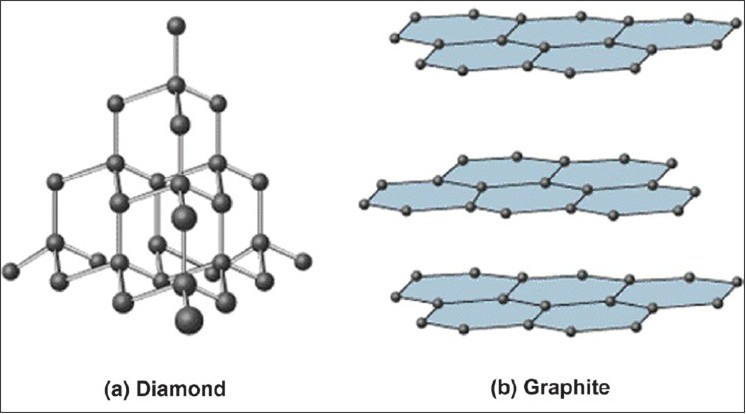
Is Carbon A Good Conductor Of Electricity. Oxidized silver is not as good a conductor as untarnished silver. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. Many common applications also rely on one or more beneficial properties such as the fact that it is a good thermal conductor or has low reactivity reaction with water and acids. Graphite is an interesting material an allotrope of carbon as is diamond.
Why Is Graphite Considered As A Non Metal Even Though It Is A Good Conductor Of Electricity Quora From quora.com
If there are different phases of a material conductivity will slow slightly at the interface and may be different from one structure than another. Crystal structure and phases. However like a metal graphite is a very good conductor of. Silver is not always an ideal choice as a material however because it is expensive and susceptible to tarnishing and the oxide layer known as tarnish is not conductive. It displays properties of both metals and nonmetals. Graphite contains only carbon atoms.
The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver.
Carbon atoms has 4 valence electrons outermost electrons each carbon atom links to 3 neighboring carbon atoms by single covalent bond leaving fourth electron free. Are good conductors of electricity ii are poor conductors of electricity iii have strong forces of attraction between their molecules. However like a metal graphite is a very good conductor of. It displays properties of both metals and nonmetals. Graphite contains only carbon atoms. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver.
 Source: quora.com
Source: quora.com
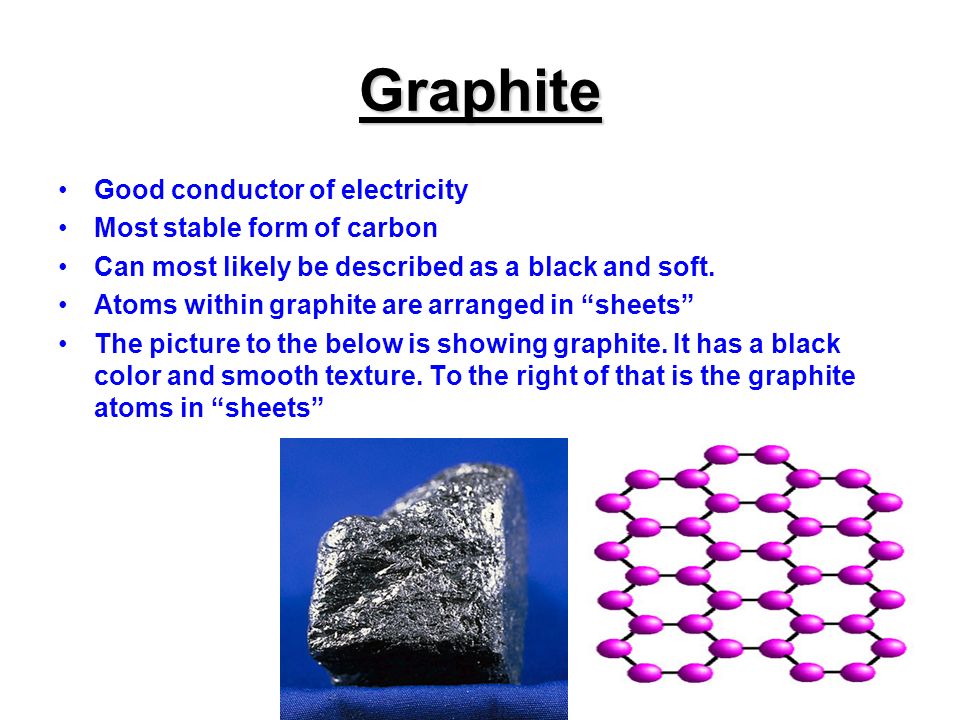
Graphite is structured into planes with tightly bound atoms. Graphite is a good conductor of electricity because its electrons are delocalized or free to move around. Oxidized silver is not as good a conductor as untarnished silver. Graphite is an interesting material an allotrope of carbon as is diamond. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver.
Source: quora.com
The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. 10 electrical conductors. However like a metal graphite is a very good conductor of. Silver is not always an ideal choice as a material however because it is expensive and susceptible to tarnishing and the oxide layer known as tarnish is not conductive. There is a great deal of distance between planes and they are bonded weakly together allowing the electrons to move around.
 Source: toppr.com
Source: toppr.com
There is a great deal of distance between planes and they are bonded weakly together allowing the electrons to move around. Are good conductors of electricity ii are poor conductors of electricity iii have strong forces of attraction between their molecules. If there are different phases of a material conductivity will slow slightly at the interface and may be different from one structure than another. Because copper is an excellent electrical conductor most of its common uses are for electrical purposes. 10 electrical conductors.

Graphite is a good conductor of electricity because its electrons are delocalized or free to move around. There is a great deal of distance between planes and they are bonded weakly together allowing the electrons to move around. The allotrope of carbon which is a good conductor of heat and electricity is a diamond b graphite c charcoal d none of these. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. Graphite is structured into planes with tightly bound atoms.
Source: quora.com
However like a metal graphite is a very good conductor of. Graphite is a good conductor of electricity because its electrons are delocalized or free to move around. Carbon atoms has 4 valence electrons outermost electrons each carbon atom links to 3 neighboring carbon atoms by single covalent bond leaving fourth electron free. Many common applications also rely on one or more beneficial properties such as the fact that it is a good thermal conductor or has low reactivity reaction with water and acids. If there are different phases of a material conductivity will slow slightly at the interface and may be different from one structure than another.
 Source: thoughtco.com
Source: thoughtco.com
Oxidized silver is not as good a conductor as untarnished silver. If there are different phases of a material conductivity will slow slightly at the interface and may be different from one structure than another. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. It displays properties of both metals and nonmetals. Because copper is an excellent electrical conductor most of its common uses are for electrical purposes.
Source: quora.com
Graphite is an interesting material an allotrope of carbon as is diamond. Graphite is a good conductor of electricity because its electrons are delocalized or free to move around. Graphite is structured into planes with tightly bound atoms. Oxidized silver is not as good a conductor as untarnished silver. Silver is not always an ideal choice as a material however because it is expensive and susceptible to tarnishing and the oxide layer known as tarnish is not conductive.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Graphite carbon is the only non metal which is good conductor of electricity. Some of the common uses of copper include. Thus the free electron is able to conduct electricity in graphite. Graphite is structured into planes with tightly bound atoms. 10 electrical conductors.
 Source: brainly.in
Source: brainly.in
Graphite is structured into planes with tightly bound atoms. There is a great deal of distance between planes and they are bonded weakly together allowing the electrons to move around. Graphite carbon is the only non metal which is good conductor of electricity. Silver is not always an ideal choice as a material however because it is expensive and susceptible to tarnishing and the oxide layer known as tarnish is not conductive. If there are different phases of a material conductivity will slow slightly at the interface and may be different from one structure than another.
 Source: brainly.in
Source: brainly.in
10 electrical conductors. Are good conductors of electricity ii are poor conductors of electricity iii have strong forces of attraction between their molecules. Crystal structure and phases. Graphite is structured into planes with tightly bound atoms. Graphite is a good conductor of electricity because its electrons are delocalized or free to move around.
 Source: zigya.com
Source: zigya.com
Crystal structure and phases. Impurities hinder electron flow. Graphite is structured into planes with tightly bound atoms. The best electrical conductor under conditions of ordinary temperature and pressure is the metallic element silver. The allotrope of carbon which is a good conductor of heat and electricity is a diamond b graphite c charcoal d none of these.
 Source: quora.com
Source: quora.com
Are good conductors of electricity ii are poor conductors of electricity iii have strong forces of attraction between their molecules. There is a great deal of distance between planes and they are bonded weakly together allowing the electrons to move around. Because copper is an excellent electrical conductor most of its common uses are for electrical purposes. Graphite is an interesting material an allotrope of carbon as is diamond. Are good conductors of electricity ii are poor conductors of electricity iii have strong forces of attraction between their molecules.
 Source: slideplayer.com
Source: slideplayer.com
The allotrope of carbon which is a good conductor of heat and electricity is a diamond b graphite c charcoal d none of these. Thus the free electron is able to conduct electricity in graphite. There is a great deal of distance between planes and they are bonded weakly together allowing the electrons to move around. It displays properties of both metals and nonmetals. However like a metal graphite is a very good conductor of.
 Source: pt.slideshare.net
Source: pt.slideshare.net
Graphite contains only carbon atoms. 10 electrical conductors. Graphite carbon is the only non metal which is good conductor of electricity. However like a metal graphite is a very good conductor of. The allotrope of carbon which is a good conductor of heat and electricity is a diamond b graphite c charcoal d none of these.
Source: quora.com
Carbon atoms has 4 valence electrons outermost electrons each carbon atom links to 3 neighboring carbon atoms by single covalent bond leaving fourth electron free. It displays properties of both metals and nonmetals. Some of the common uses of copper include. 10 electrical conductors. Carbon atoms has 4 valence electrons outermost electrons each carbon atom links to 3 neighboring carbon atoms by single covalent bond leaving fourth electron free.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title is carbon a good conductor of electricity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.