Hydrolysis of water lab
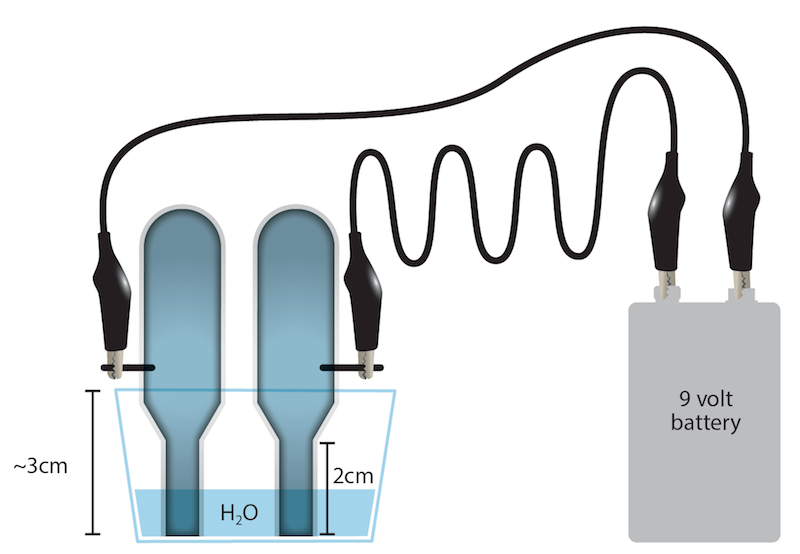
Hydrolysis Of Water Lab. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. The experiment was then repeated with 40 60 v v isopropanol water mixture and a larger value of k 0 0007 s 1 was obtained. In acidic and basic solutions we have considered the h and oh ions formed directly from acids and bases by the process called ionization. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts.
 Pin On Stem From pinterest.com
Pin On Stem From pinterest.com
In acidic and basic solutions we have considered the h and oh ions formed directly from acids and bases by the process called ionization. Sometimes called water splitting electrolysis requires a minimum potential difference of 1 23 volts. Water is a simple chemical made from two gases hydrogen and oxygen. Every molecule of water has two atoms of hydrogen and one atom of oxygen. The concentration of the water is a lot. We will observe the results of hydrolysis and determine which salt solutions are neutral acidic or basic.
Hydrolysis is any chemical reaction in which a molecule of water ruptures one or more chemical bonds.
This lab will explore the acid or base character of different salts dissolved in water. We will observe the results of hydrolysis and determine which salt solutions are neutral acidic or basic. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. When a carbohydrate is broken into its component sugar molecules by hydrolysis this is recognized as saccharification. Water is a simple chemical made from two gases hydrogen and oxygen. Well and there would be a large amount of water.
 Source: youtube.com
Source: youtube.com
That is exactly what happens when esters are hydrolysed by water or by dilute acids such as dilute hydrochloric acid. In freshwater hydroxide ion and water are the dominant nucleophiles with oh being about 10 000 times more reactive than h2o in substitution at carbon 1 2. A common type of hydrolysis occurs when a salt of a weak acid or weak base or both is dissolved in water. A solvent so we would not be able to see the difference of the water concentration from the. We know water is.
 Source: pinterest.com
Source: pinterest.com
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Water is a simple chemical made from two gases hydrogen and oxygen. When a carbohydrate is broken into its component sugar molecules by hydrolysis this is recognized as saccharification. The hydrolysis of a. The value of the rate constant k obtained was 0 0002 s 1.
 Source: orbitingfrog.com
Source: orbitingfrog.com
This lab may be used to teach or reinforce many concepts including. The term is used broadly for substitution elimination and solvation reactions in which water is the nucleophile. In acidic and basic solutions we have considered the h and oh ions formed directly from acids and bases by the process called ionization. The concentration of the water is a lot. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts.
 Source: orbitingfrog.com
Source: orbitingfrog.com
In chemistry acid hydrolysis is a process in which a protic acid is used to catalyze the cleavage of a chemical bond via a nucleophile substitution reaction with the addition of the elements of water h2 o. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. We will observe the results of hydrolysis and determine which salt solutions are neutral acidic or basic. Every molecule of water has two atoms of hydrogen and one atom of oxygen. The value of the rate constant k obtained was 0 0002 s 1.
 Source: youtube.com
Source: youtube.com
Hydrolysis occurs when salt from a weak base or acid dissolves in liquid. That is exactly what happens when esters are hydrolysed by water or by dilute acids such as dilute hydrochloric acid. Hydrolysis is any chemical reaction in which a molecule of water ruptures one or more chemical bonds. What this means is that if the mechanism. Technically hydrolysis is a reaction with water.
 Source: manoa.hawaii.edu
Source: manoa.hawaii.edu
The value of the rate constant k obtained was 0 0002 s 1. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. Water is a simple chemical made from two gases hydrogen and oxygen. In acidic and basic solutions we have considered the h and oh ions formed directly from acids and bases by the process called ionization. The term is used broadly for substitution elimination and solvation reactions in which water is the nucleophile.
 Source: kidslovekits.com
Source: kidslovekits.com
The term is used broadly for substitution elimination and solvation reactions in which water is the nucleophile. Provided form the lab manual had water. Every molecule of water has two atoms of hydrogen and one atom of oxygen. That is exactly what happens when esters are hydrolysed by water or by dilute acids such as dilute hydrochloric acid. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts.
 Source: upsbatterycenter.com
Source: upsbatterycenter.com
Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. Every molecule of water has two atoms of hydrogen and one atom of oxygen. The value of the rate constant k obtained was 0 0002 s 1. In this case the water molecule would give away a proton. The concentration of the water is a lot.
 Source: m.youtube.com
Source: m.youtube.com
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. The hydrolysis of a. Every molecule of water has two atoms of hydrogen and one atom of oxygen. That is exactly what happens when esters are hydrolysed by water or by dilute acids such as dilute hydrochloric acid.
 Source: thejoysharing.com
Source: thejoysharing.com
Water is a simple chemical made from two gases hydrogen and oxygen. A solvent so we would not be able to see the difference of the water concentration from the. What this means is that if the mechanism. The experiment was then repeated with 40 60 v v isopropanol water mixture and a larger value of k 0 0007 s 1 was obtained. A common type of hydrolysis occurs when a salt of a weak acid or weak base or both is dissolved in water.
 Source: thejoysharing.com
Source: thejoysharing.com
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. The hydrolysis of a. In this case the water molecule would give away a proton. This is the most common type of hydrolysis. Well and there would be a large amount of water.
 Source: chemdemos.uoregon.edu
Source: chemdemos.uoregon.edu
In acidic and basic solutions we have considered the h and oh ions formed directly from acids and bases by the process called ionization. A common type of hydrolysis occurs when a salt of a weak acid or weak base or both is dissolved in water. When this occurs water spontaneously ionizes into hydroxide anions and hydronium cations. Hydrolysis is any chemical reaction in which a molecule of water ruptures one or more chemical bonds. The experiment was then repeated with 40 60 v v isopropanol water mixture and a larger value of k 0 0007 s 1 was obtained.
 Source: youtube.com
Source: youtube.com
Hydrolysis is any chemical reaction in which a molecule of water ruptures one or more chemical bonds. That is exactly what happens when esters are hydrolysed by water or by dilute acids such as dilute hydrochloric acid. The value of the rate constant k obtained was 0 0002 s 1. Hydrolysis reaction with water is usually the most important reaction for molecules susceptible to nucleophilic attack. Every molecule of water has two atoms of hydrogen and one atom of oxygen.
 Source: docbrown.info
Source: docbrown.info
The term is used broadly for substitution elimination and solvation reactions in which water is the nucleophile. The experiment was then repeated with 40 60 v v isopropanol water mixture and a larger value of k 0 0007 s 1 was obtained. That is exactly what happens when esters are hydrolysed by water or by dilute acids such as dilute hydrochloric acid. Provided form the lab manual had water. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas.
 Source: learning-center.homesciencetools.com
Source: learning-center.homesciencetools.com
A solvent so we would not be able to see the difference of the water concentration from the. Hydrolysis is any chemical reaction in which a molecule of water ruptures one or more chemical bonds. The experiment was then repeated with 40 60 v v isopropanol water mixture and a larger value of k 0 0007 s 1 was obtained. With this lab students can use electricity to separate the water back into hydrogen and oxygen. The hydrolysis of a.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title hydrolysis of water lab by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.