How to make galvanic cell
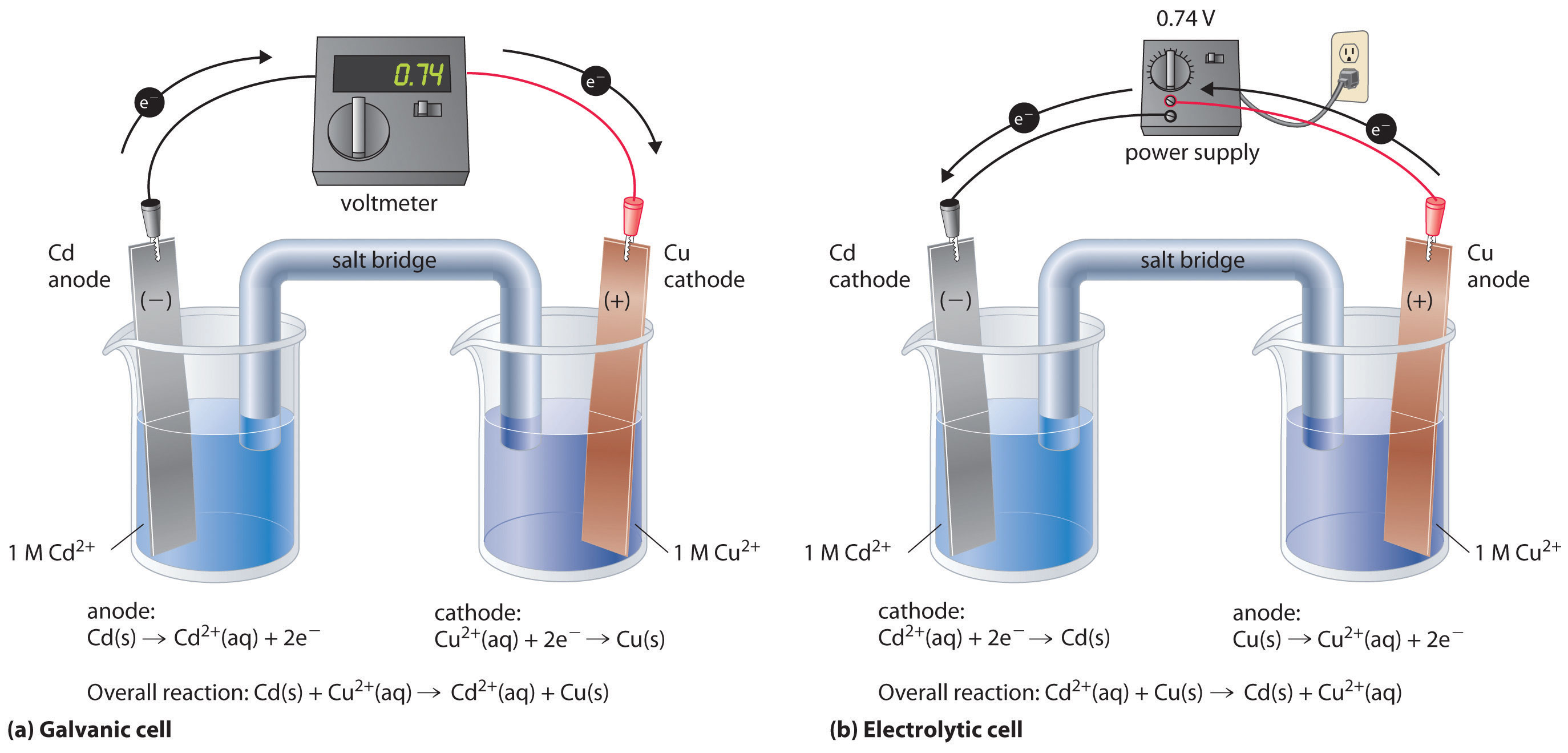
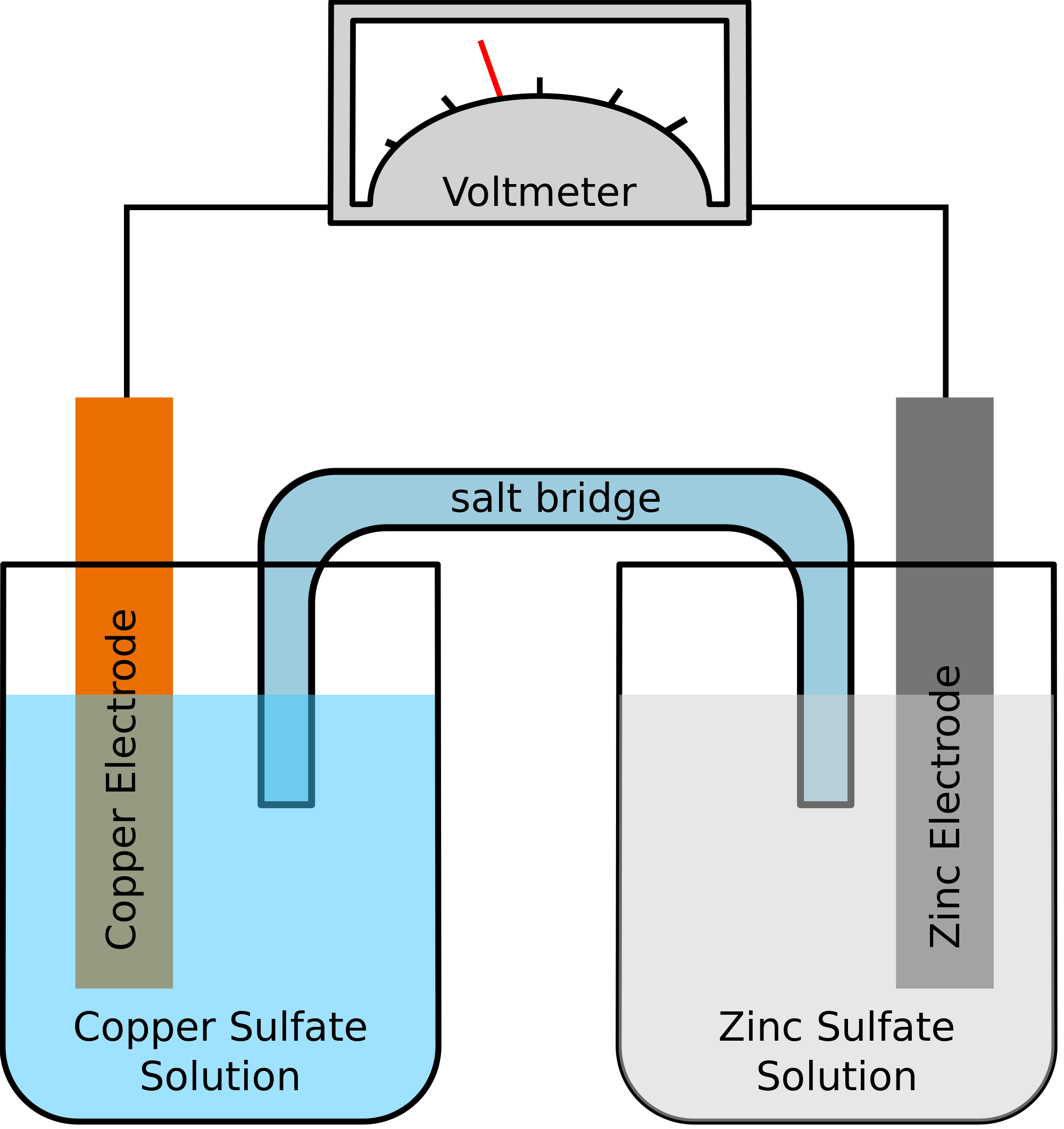
How To Make Galvanic Cell. Cu 2 2e cu. In both galvanic and electrolytic cells oxidation takes place at the anode and electrons flow from the anode to the cathode. Reactions of daniel cell at cathode and anode are. The two metal strips are connected by a wire that allows electricity to flow and the beakers are connected by a salt bridge.
Galvanic Cell Wikipedia From en.wikipedia.org
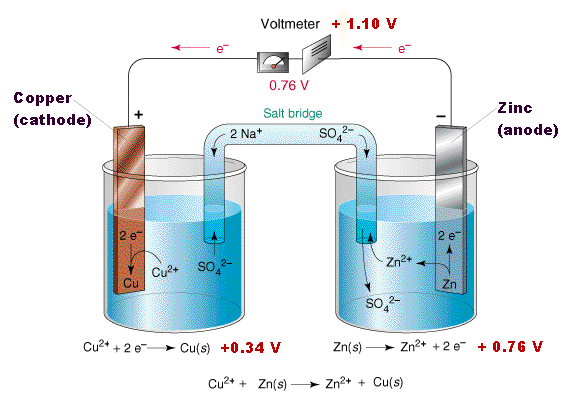
Galvanic or voltaic cells. The two metal strips are connected by a wire that allows electricity to flow and the beakers are connected by a salt bridge. Reactions of daniel cell at cathode and anode are. This galvanic cell which contains only three parts can be assembled in a few minutes. About press copyright contact us creators advertise developers terms privacy policy safety how youtube works test new features press copyright contact us creators. In daniel s cell copper ions are reduced at the cathode while zinc is oxidized at the anode.
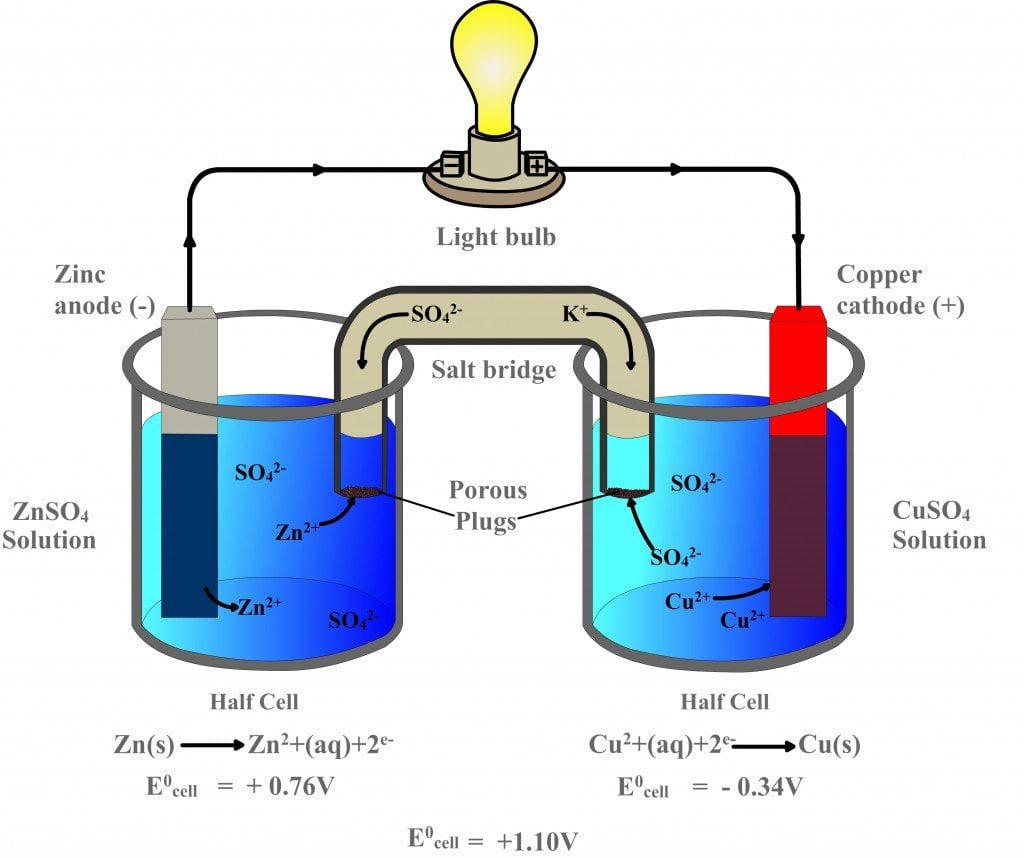
The reaction of metallic zinc with aqueous copper ii ions in a galvanic cell.
The cathode of a galvanic cell is its positive terminal. The two metal strips are connected by a wire that allows electricity to flow and the beakers are connected by a salt bridge. The principle is simple and 1st time discovered in 1780 by luigi galvani in the famous frog leg experiment if you like to know more about it check wiki. Soaking string cotton or paper in an electrolyte solution. This gonna be short and simple instruction for making a galvanic cell and battery from some normal wood screws or nails or any other similar material and copper wire and some tissues and stickies and using the battery to power an led. For this reason galvanic cells are commonly used as batteries.
 Source: socratic.org
Source: socratic.org
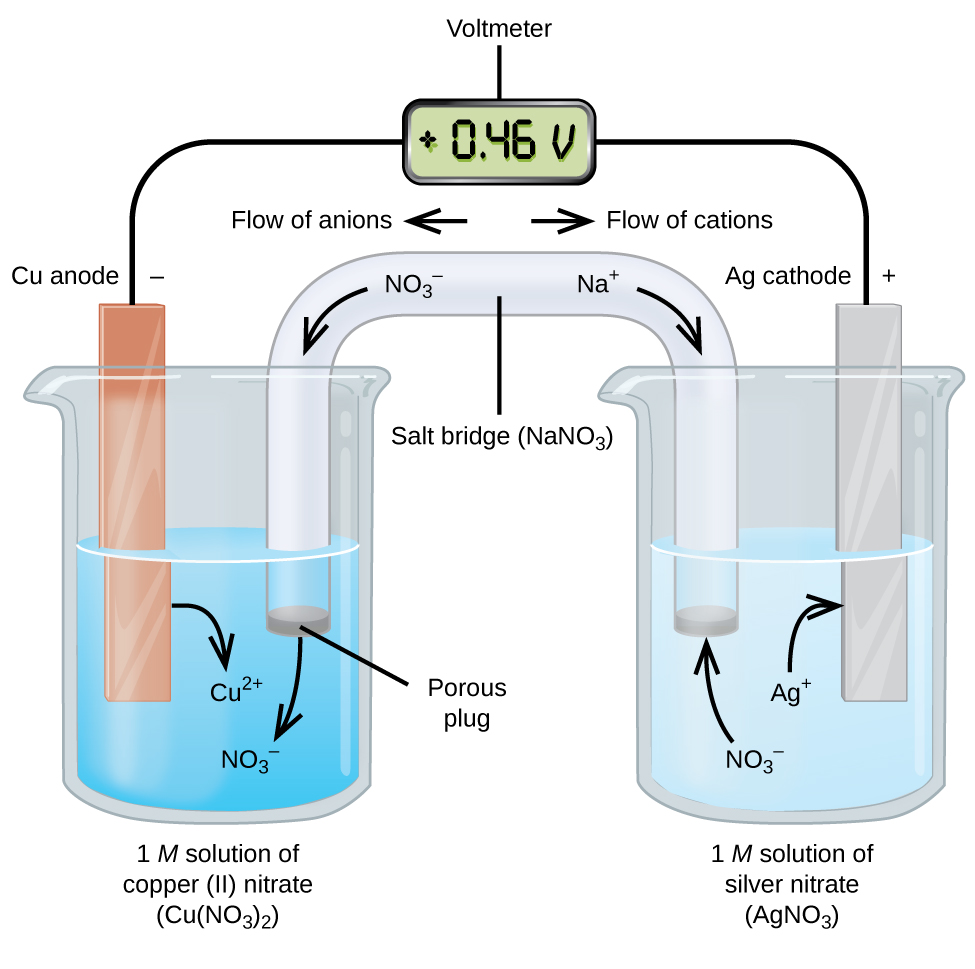
A a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 ions and a zinc strip into a different beaker that contains an aqueous 1 m solution of zn 2 ions. One of these electrodes the cathode shall be a positively charged electrode while the other shall be the anode the negatively charged electrode. Galvanic or voltaic cells. The reaction of metallic zinc with aqueous copper ii ions in a galvanic cell. For this reason galvanic cells are commonly used as batteries.
 Source: youtube.com
Source: youtube.com
This gonna be short and simple instruction for making a galvanic cell and battery from some normal wood screws or nails or any other similar material and copper wire and some tissues and stickies and using the battery to power an led. For this reason galvanic cells are commonly used as batteries. The two metal strips are connected by a wire that allows electricity to flow and the beakers are connected by a salt bridge. Galvanic or voltaic cells. Batteries or single galvanic cells have been designed for educational purposes 1 5 this article describes how students can make a simple galvanic cell and use it to light a light emitting diode led.
 Source: chem.libretexts.org
Source: chem.libretexts.org
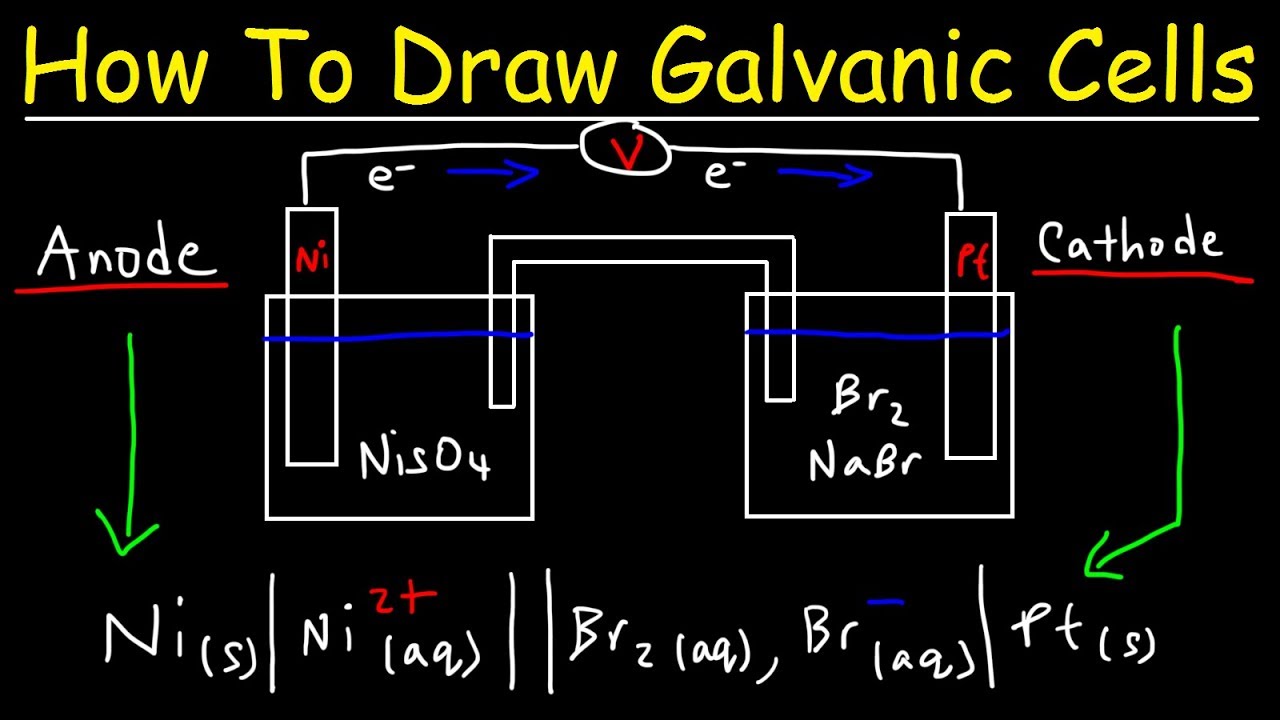
Setup of a galvanic cell in order to create a galvanic cell one would have to go through the following setup. Zn zn 2 2e. The two metal strips are connected by a wire that allows electricity to flow and the beakers are connected by a salt bridge. One of these electrodes the cathode shall be a positively charged electrode while the other shall be the anode the negatively charged electrode. The redox reaction in a galvanic cell is a spontaneous reaction.
 Source: socratic.org
Source: socratic.org
The reaction of metallic zinc with aqueous copper ii ions in a galvanic cell. Zn zn 2 2e. The reaction of metallic zinc with aqueous copper ii ions in a galvanic cell. This gonna be short and simple instruction for making a galvanic cell and battery from some normal wood screws or nails or any other similar material and copper wire and some tissues and stickies and using the battery to power an led. In daniel s cell copper ions are reduced at the cathode while zinc is oxidized at the anode.
 Source: scienceabc.com
Source: scienceabc.com
The reaction of metallic zinc with aqueous copper ii ions in a galvanic cell. Cu 2 2e cu. Galvanic or voltaic cells. Setup of a galvanic cell in order to create a galvanic cell one would have to go through the following setup. This gonna be short and simple instruction for making a galvanic cell and battery from some normal wood screws or nails or any other similar material and copper wire and some tissues and stickies and using the battery to power an led.
 Source: thoughtco.com
Source: thoughtco.com
The cathode of a galvanic cell is its positive terminal. Setup of a galvanic cell in order to create a galvanic cell one would have to go through the following setup. The reaction of metallic zinc with aqueous copper ii ions in a galvanic cell. Occasionally fritted glass porous glass though not a salt bridge is used to connect two half cells. Batteries or single galvanic cells have been designed for educational purposes 1 5 this article describes how students can make a simple galvanic cell and use it to light a light emitting diode led.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Obtain a peice of material that is large enough to reach between two half cells. Zn zn 2 2e. Galvanic cells also known as voltaic cells are electrochemical cells in which spontaneous oxidation reduction reactions produce electrical energy in writing the equations it is often convenient to separate the oxidation reduction reactions into half reactions to facilitate balancing the overall equation and to emphasize the actual chemical transformations. The two metal strips are connected by a wire that allows electricity to flow and the beakers are connected by a salt bridge. About press copyright contact us creators advertise developers terms privacy policy safety how youtube works test new features press copyright contact us creators.
 Source: youtube.com
Source: youtube.com
About press copyright contact us creators advertise developers terms privacy policy safety how youtube works test new features press copyright contact us creators. Soaking string cotton or paper in an electrolyte solution. Zn zn 2 2e. Reactions of daniel cell at cathode and anode are. Occasionally fritted glass porous glass though not a salt bridge is used to connect two half cells.
 Source: toppr.com
Source: toppr.com
Zn zn 2 2e. This gonna be short and simple instruction for making a galvanic cell and battery from some normal wood screws or nails or any other similar material and copper wire and some tissues and stickies and using the battery to power an led. Setup of a galvanic cell in order to create a galvanic cell one would have to go through the following setup. In daniel s cell copper ions are reduced at the cathode while zinc is oxidized at the anode. Galvanic or voltaic cells.
 Source: toppr.com
Source: toppr.com
Cu 2 2e cu. Soaking string cotton or paper in an electrolyte solution. Obtain a peice of material that is large enough to reach between two half cells. The reaction of metallic zinc with aqueous copper ii ions in a galvanic cell. For this reason galvanic cells are commonly used as batteries.
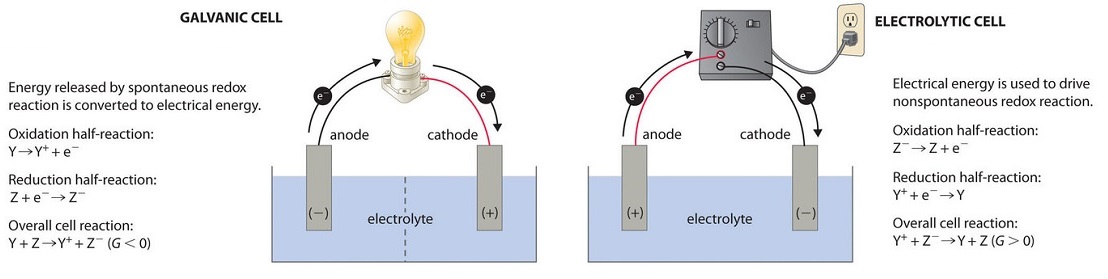 Source: chem.libretexts.org
Source: chem.libretexts.org
One of these electrodes the cathode shall be a positively charged electrode while the other shall be the anode the negatively charged electrode. Zn zn 2 2e. Obtain a peice of material that is large enough to reach between two half cells. For this reason galvanic cells are commonly used as batteries. The cathode of a galvanic cell is its positive terminal.
 Source: intl.siyavula.com
Source: intl.siyavula.com
In daniel s cell copper ions are reduced at the cathode while zinc is oxidized at the anode. The reaction of metallic zinc with aqueous copper ii ions in a galvanic cell. This gonna be short and simple instruction for making a galvanic cell and battery from some normal wood screws or nails or any other similar material and copper wire and some tissues and stickies and using the battery to power an led. The redox reaction in a galvanic cell is a spontaneous reaction. A a galvanic cell can be constructed by inserting a copper strip into a beaker that contains an aqueous 1 m solution of cu 2 ions and a zinc strip into a different beaker that contains an aqueous 1 m solution of zn 2 ions.
Source: en.wikipedia.org
In both galvanic and electrolytic cells oxidation takes place at the anode and electrons flow from the anode to the cathode. Reactions of daniel cell at cathode and anode are. Galvanic or voltaic cells. Soaking string cotton or paper in an electrolyte solution. In daniel s cell copper ions are reduced at the cathode while zinc is oxidized at the anode.
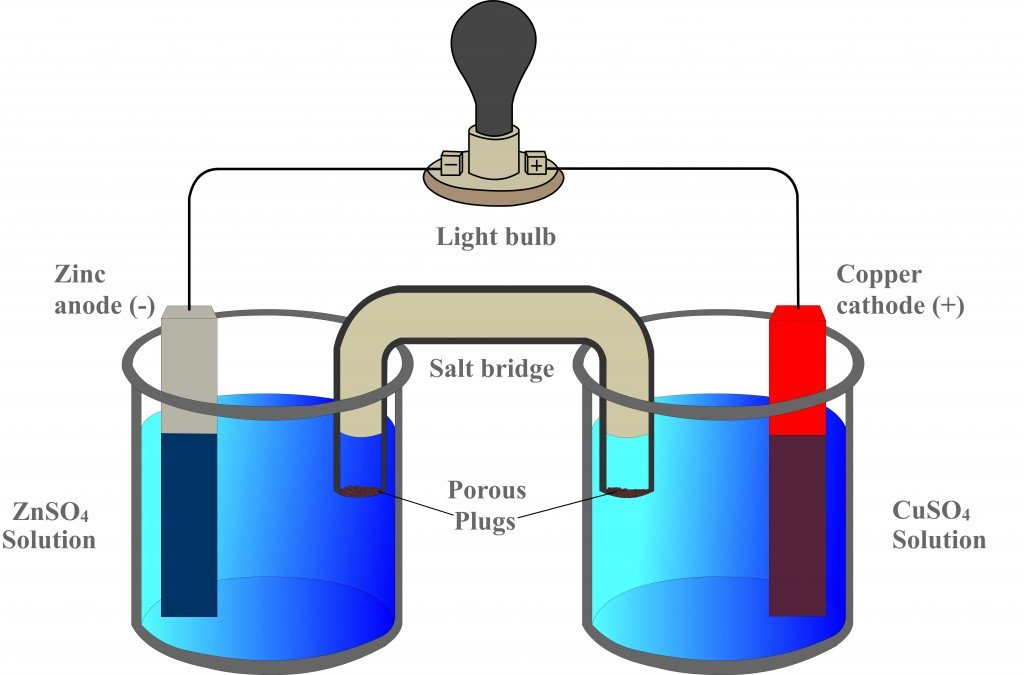 Source: scienceabc.com
Source: scienceabc.com
The principle is simple and 1st time discovered in 1780 by luigi galvani in the famous frog leg experiment if you like to know more about it check wiki. The redox reaction in a galvanic cell is a spontaneous reaction. The principle is simple and 1st time discovered in 1780 by luigi galvani in the famous frog leg experiment if you like to know more about it check wiki. Galvanic or voltaic cells. This galvanic cell which contains only three parts can be assembled in a few minutes.
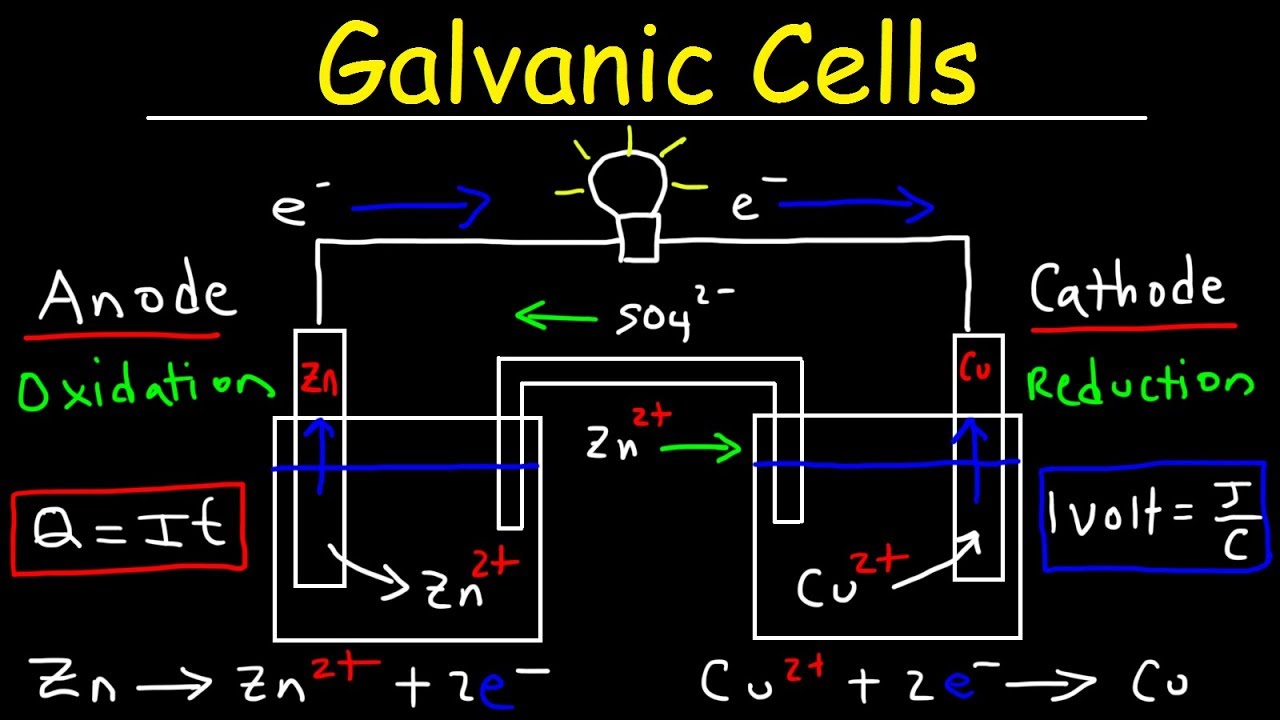 Source: youtube.com
Source: youtube.com
The redox reaction in a galvanic cell is a spontaneous reaction. Obtain a peice of material that is large enough to reach between two half cells. Reactions of daniel cell at cathode and anode are. Setup of a galvanic cell in order to create a galvanic cell one would have to go through the following setup. Zn zn 2 2e.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to make galvanic cell by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.