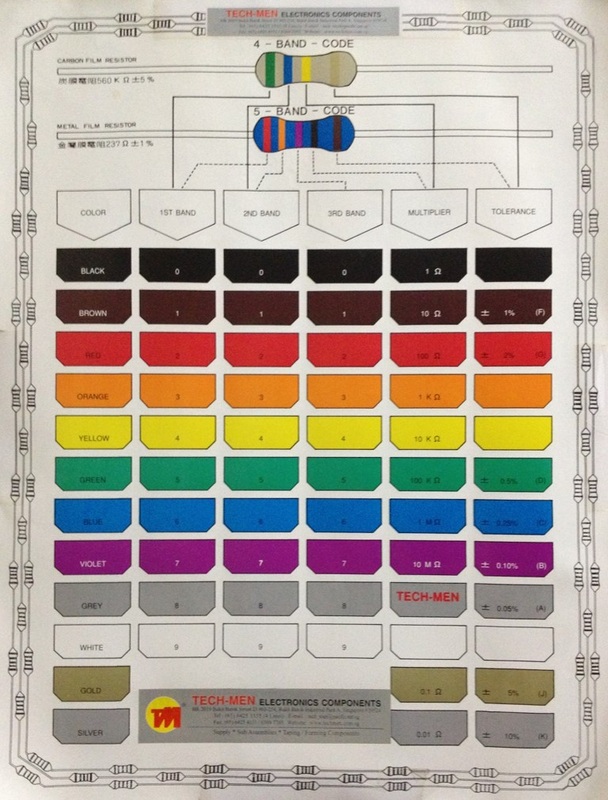
How to make copper plating solution
How To Make Copper Plating Solution. You may be on to something with the plating of the penny making it easier but the issue is that once the top layer of copper is gone from the penny the other metals inside of the penny zinc will start to form ions in the solution and then eventually plate the object with that metal. 25 oz weight or 1 6 cups volume 98 sulfuric acid to 3 quarts 12 cups of cold deionized. Afterwards you can pour the vinegar down the drain and trash the sponge. If you don t want to buy that then all you can do if minimize immersion plating by use of a dilute solution and hot entry.
 Choosing And Troubleshooting Copper Electroplating Processes Products Finishing From pfonline.com
Choosing And Troubleshooting Copper Electroplating Processes Products Finishing From pfonline.com
Electrolyze this solution for 10 minutes or so. M l 1 ρ o f h 2 s o 4 271 m l l. You may be on to something with the plating of the penny making it easier but the issue is that once the top layer of copper is gone from the penny the other metals inside of the penny zinc will start to form ions in the solution and then eventually plate the object with that metal. The proprietary plating solution would be best for the reason described. Please check if i am correct with calculation. It seems to take a few minutes for some of the copper metal to dissolve and be available for plating.
Copper electroplating requires electricty.
100 g l 5 l 1 84 g. It seems to take a few minutes for some of the copper metal to dissolve and be available for plating. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. Plating can be tricky but this method comes as close. Screw on the lid and gently swirl the liquid and copper in the jar. Specifically you want 10 to 12 oz of copper sulfate crystals per gallon of plating solution.
 Source: youtube.com
Source: youtube.com
Then use 37 h c l. 200 g 5 1000 g. 0 05 g l 50 p p m 5 l 1 19 g m l ρ of h c l 0 21 m l l. 100 g l 5 l 1 84 g. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this.
 Source: researchgate.net
Source: researchgate.net
M l 1 ρ o f h 2 s o 4 271 m l l. 100 g l 5 l 1 84 g. The solution will heat up very rapidly as you add the acid so be very careful. 200 g 5 1000 g. This is probably the easiest way to create reliable attractive copper plate on many different metals.
 Source: m.youtube.com
Source: m.youtube.com
You may be on to something with the plating of the penny making it easier but the issue is that once the top layer of copper is gone from the penny the other metals inside of the penny zinc will start to form ions in the solution and then eventually plate the object with that metal. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. The solution will heat up very rapidly as you add the acid so be very careful. As time passes the mixture will become more and more blue. 0 05 g l 50 p p m 5 l 1 19 g m l ρ of h c l 0 21 m l l.
 Source: m.youtube.com
Source: m.youtube.com
0 05 g l 50 p p m 5 l 1 19 g m l ρ of h c l 0 21 m l l. It seems to take a few minutes for some of the copper metal to dissolve and be available for plating. 30 60sec will give you a dark copper color finish. Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin. Copper electroplating requires electricty.
 Source: bytechlab.com
Source: bytechlab.com
You can t stop it. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. The proprietary plating solution would be best for the reason described. M l 1 ρ o f h 2 s o 4 271 m l l. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this.
 Source: rfcafe.com
Source: rfcafe.com
Attach the positive terminal to the anode the copper wire and the negative terminal to the cathode the coin. Specifically you want 10 to 12 oz of copper sulfate crystals per gallon of plating solution. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. It avoids immersion plating. Electrolyze this solution for 10 minutes or so.
 Source: spark.iop.org
Source: spark.iop.org
Then try a stronger solution of copper sulphate and sulphuric acid for the balance of the plating. Afterwards you can pour the vinegar down the drain and trash the sponge. As time passes the mixture will become more and more blue. Electrolyze this solution for 10 minutes or so. Please check if i am correct with calculation.
 Source: m.youtube.com
Source: m.youtube.com
As time passes the mixture will become more and more blue. If you don t want to buy that then all you can do if minimize immersion plating by use of a dilute solution and hot entry. 200 g 5 1000 g. Copper electroplating requires electricty. M l 1 ρ o f h 2 s o 4 271 m l l.
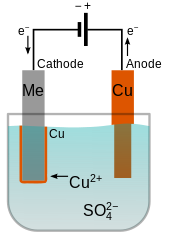 Source: en.wikipedia.org
Source: en.wikipedia.org
Afterwards you can pour the vinegar down the drain and trash the sponge. 0 05 g l 50 p p m 5 l 1 19 g m l ρ of h c l 0 21 m l l. Then use 37 h c l. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. It avoids immersion plating.
 Source: chemedx.org
Source: chemedx.org
The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. You can t stop it. The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. Afterwards you can pour the vinegar down the drain and trash the sponge. 100 g l 5 l 1 84 g.
 Source: youtube.com
Source: youtube.com
Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. To make up a single gallon of acid copper electrolyte add. Plating can be tricky but this method comes as close. Afterwards you can pour the vinegar down the drain and trash the sponge. M l 1 ρ o f h 2 s o 4 271 m l l.
 Source: advancedplatingtech.com
Source: advancedplatingtech.com
To make up a single gallon of acid copper electrolyte add. If you don t want to buy that then all you can do if minimize immersion plating by use of a dilute solution and hot entry. 200 g 5 1000 g. Now place the copper into the warm vinegar peroxide mixture. To make up a single gallon of acid copper electrolyte add.
 Source: pfonline.com
Source: pfonline.com
The proprietary plating solution would be best for the reason described. Then try a stronger solution of copper sulphate and sulphuric acid for the balance of the plating. Electrolyze this solution for 10 minutes or so. Specifically you want 10 to 12 oz of copper sulfate crystals per gallon of plating solution. When you are done plating drop the sponge into your solution overnight to remove cu2 ions very bad for the environment from the vinegar before you dispose of it.
 Source: m.youtube.com
Source: m.youtube.com
Specifically you want 10 to 12 oz of copper sulfate crystals per gallon of plating solution. 30 60sec will give you a dark copper color finish. 25 oz weight or 1 6 cups volume 98 sulfuric acid to 3 quarts 12 cups of cold deionized. Then use 37 h c l. 200 g 5 1000 g.
 Source: instructables.com
Source: instructables.com
The copper scrubby material can be quite sharp you may want to put on a pair of gloves to do this. 100 g l 5 l 1 84 g. Welcome to science at home in this experiment we are exploring copper electroplating by coating a nail with copper. It avoids immersion plating. You can t stop it.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to make copper plating solution by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.