How to make benedict solution
How To Make Benedict Solution. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. How to make your own benedict s solution. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves.
 Benedict S Test Principle Reagent Preparation Procedure And Interpretation Laboratoryinfo Com From laboratoryinfo.com
Benedict S Test Principle Reagent Preparation Procedure And Interpretation Laboratoryinfo Com From laboratoryinfo.com
Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Pour 60ml of water into beaker place on magnetic stirrer and switch on. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. How to make your own benedict s solution. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate.
How to make your own benedict s solution.
Pour 60ml of water into beaker place on magnetic stirrer and switch on. How to make your own benedict s solution. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Pour 60ml of water into beaker place on magnetic stirrer and switch on. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate.
 Source: walmart.com
Source: walmart.com
Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. How to make your own benedict s solution. Pour 60ml of water into beaker place on magnetic stirrer and switch on. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution.
 Source: m.youtube.com
Source: m.youtube.com
Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Pour 60ml of water into beaker place on magnetic stirrer and switch on. How to make your own benedict s solution. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate.
 Source: mystrica.com
Source: mystrica.com
Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. How to make your own benedict s solution. Pour 60ml of water into beaker place on magnetic stirrer and switch on.
 Source: laboratoryinfo.com
Source: laboratoryinfo.com
Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Pour 60ml of water into beaker place on magnetic stirrer and switch on. How to make your own benedict s solution.
 Source: igbiologyy.blogspot.com
Source: igbiologyy.blogspot.com
Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Pour 60ml of water into beaker place on magnetic stirrer and switch on. How to make your own benedict s solution.
 Source: homesciencetools.com
Source: homesciencetools.com
Pour 60ml of water into beaker place on magnetic stirrer and switch on. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. How to make your own benedict s solution. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution.
 Source: byjus.com
Source: byjus.com
Pour 60ml of water into beaker place on magnetic stirrer and switch on. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Pour 60ml of water into beaker place on magnetic stirrer and switch on. How to make your own benedict s solution. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Pour 60ml of water into beaker place on magnetic stirrer and switch on. How to make your own benedict s solution.
 Source: brilliantbiologystudent.weebly.com
Source: brilliantbiologystudent.weebly.com
Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. How to make your own benedict s solution. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. Pour 60ml of water into beaker place on magnetic stirrer and switch on.
 Source: microbiologyinfo.com
Source: microbiologyinfo.com
Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. How to make your own benedict s solution. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution.
 Source: sciencecompany.com
Source: sciencecompany.com
Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Pour 60ml of water into beaker place on magnetic stirrer and switch on. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. How to make your own benedict s solution.
 Source: microbenotes.com
Source: microbenotes.com
How to make your own benedict s solution. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. How to make your own benedict s solution. Pour 60ml of water into beaker place on magnetic stirrer and switch on. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution.
 Source: youtube.com
Source: youtube.com
Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. How to make your own benedict s solution.
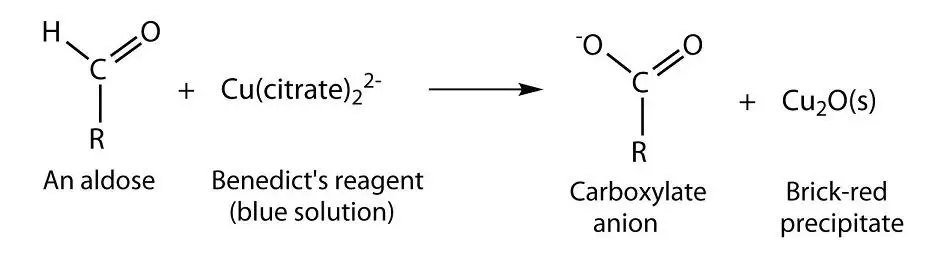 Source: laboratoryinfo.com
Source: laboratoryinfo.com
How to make your own benedict s solution. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate. How to make your own benedict s solution. Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. Pour 60ml of water into beaker place on magnetic stirrer and switch on.
 Source: philipharris.co.uk
Source: philipharris.co.uk
Add 10g of anhydrous sodium carbonate and continue stirring until the solid dissolves. How to make your own benedict s solution. Pour 60ml of water into beaker place on magnetic stirrer and switch on. Add 17 0g of trisodium citrate 2 water and 1 74g of copper ii sulfate 5 water to the sodium carbonate solution. Benedict s solution or benedict s reagent can be prepared by complexing cupric ions cu2 cations from the copper sulfate pentahydrate with citric acid molecules in a basic environment provided by sodium carbonate.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to make benedict solution by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.